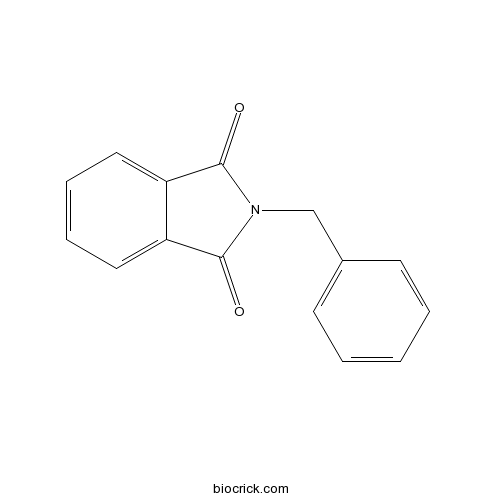
N-BenzylphthalimideCAS# 2142-01-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2142-01-0 | SDF | Download SDF |
| PubChem ID | 75059 | Appearance | Powder |
| Formula | C15H11NO2 | M.Wt | 237.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-benzylisoindole-1,3-dione | ||
| SMILES | C1=CC=C(C=C1)CN2C(=O)C3=CC=CC=C3C2=O | ||
| Standard InChIKey | WITXFYCLPDFRNM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H11NO2/c17-14-12-8-4-5-9-13(12)15(18)16(14)10-11-6-2-1-3-7-11/h1-9H,10H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

N-Benzylphthalimide Dilution Calculator

N-Benzylphthalimide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2141 mL | 21.0704 mL | 42.1408 mL | 84.2815 mL | 105.3519 mL |
| 5 mM | 0.8428 mL | 4.2141 mL | 8.4282 mL | 16.8563 mL | 21.0704 mL |
| 10 mM | 0.4214 mL | 2.107 mL | 4.2141 mL | 8.4282 mL | 10.5352 mL |
| 50 mM | 0.0843 mL | 0.4214 mL | 0.8428 mL | 1.6856 mL | 2.107 mL |
| 100 mM | 0.0421 mL | 0.2107 mL | 0.4214 mL | 0.8428 mL | 1.0535 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Picrotin
Catalog No.:BCC8233
CAS No.:21416-53-5
- 1-Decarboxy-3-oxo-ceanothic acid
Catalog No.:BCN4924
CAS No.:214150-74-0
- 26-Deoxycimicifugoside
Catalog No.:BCN2906
CAS No.:214146-75-5
- Glucoraphanin
Catalog No.:BCN3817
CAS No.:21414-41-5
- Magnoflorine
Catalog No.:BCN4923
CAS No.:2141-09-5
- CART (55-102) (human)
Catalog No.:BCC6007
CAS No.:214050-22-3
- Taxiphyllin
Catalog No.:BCN4922
CAS No.:21401-21-8
- Chrysin dimethylether
Catalog No.:BCN6799
CAS No.:21392-57-4
- Barbacarpan
Catalog No.:BCN4921
CAS No.:213912-46-0
- 1-Acetoxy-9,17-octadecadiene-12,14-diyne-11,16-diol
Catalog No.:BCN1493
CAS No.:213905-35-2
- Nifenazone
Catalog No.:BCC3822
CAS No.:2139-47-1
- Zedoarofuran
Catalog No.:BCN3527
CAS No.:213833-34-2
- 5,7-dimethoxy-2,2-dimethylchromene
Catalog No.:BCN8030
CAS No.:21421-66-9
- Demethylsuberosin
Catalog No.:BCN6508
CAS No.:21422-04-8
- Rosamultic acid
Catalog No.:BCN3516
CAS No.:214285-76-4
- 16alpha-Hydroxybauerenol
Catalog No.:BCN7724
CAS No.:214351-30-1
- 1400W dihydrochloride
Catalog No.:BCC7057
CAS No.:214358-33-5
- (+)-Syringaresinol
Catalog No.:BCN7496
CAS No.:21453-69-0
- AMT hydrochloride
Catalog No.:BCC6823
CAS No.:21463-31-0
- 2-Deacetyltaxachitriene A
Catalog No.:BCN7415
CAS No.:214769-96-7
- H-Arg(NO2)-OH
Catalog No.:BCC2864
CAS No.:2149-70-4
- Agrimonolide
Catalog No.:BCN4925
CAS No.:21499-24-1
- Bruceine D
Catalog No.:BCN2894
CAS No.:21499-66-1
- 7,3',4'-Trihydroxyflavone
Catalog No.:BCN4674
CAS No.:2150-11-0
Mechanochemical Lignin-Mediated Strecker Reaction.[Pubmed:28106742]
Molecules. 2017 Jan 17;22(1). pii: molecules22010146.
A mechanochemical Strecker reaction involving a wide range of aldehydes (aromatic, heteroaromatic and aliphatic), amines, and KCN afforded a library of alpha-aminonitriles upon mechanical activation. This multicomponent process was efficiently activated by lignocellulosic biomass as additives. Particularly, commercially available Kraft lignin was found to be the best activator for the addition of cyanide to the in situ formed imines. A comparative study of the (31)P-NMR (Nuclear Magnetic Resonance) along with IR (Infrared) data analysis for the Kraft lignin and methylated Kraft lignin samples ascertained the importance of the free hydroxyl groups in the activation of the mechanochemical reaction. The solvent-free mechanochemical Strecker reaction was then coupled with a lactamization process leading to the formation of the N-Benzylphthalimide (5a) and the isoindolinone derivative 6a.
Kinetic coupled with UV spectral evidence for near-irreversible nonionic micellar binding of N-benzylphthalimide under the typical reaction conditions: an observation against a major assumption of the pseudophase micellar model.[Pubmed:17914797]
J Phys Chem B. 2007 Oct 25;111(42):12185-94.
Pseudo-first-order rate constants (k(obs)) for alkaline hydrolysis of N-Benzylphthalimide (1) show a nonlinear decrease with the increase in [C(m)E(n)]T (total concentration of Brij 58, m = 16, n = 20 and Brij 56, m = 16, n = 10) at constant [CH(3)CN] and [NaOH]. These nonionic micellar effects, within the certain typical reaction conditions, have been explained in terms of the pseudophase micellar (PM) model. The values of micellar binding constants (KS) of 1 are 1.04 x 10(3) M(-1) (at 1.0 x 10(-3) M NaOH) and 1.08 x 10(3) M(-1) (at 2.0 x 10(-3) M NaOH) for C(16)E(20) as well as 600 M(-1) (at 7.6 x 10(-4) M NaOH) and 670 M(-1) (at 1.0 x 10(-3) M NaOH) for C(16)E(10) micelles. The pseudo-first-order rate constants (kM) for hydrolysis of 1 in C(16)E(20) micellar pseudophase are approximately 90-fold smaller than those (kW) in water phase. The values of kM for hydrolysis of 1 in C(16)E(10) micelles are almost zero. Kinetic coupled with UV spectral data reveals significant irreversible nonionic micellar binding of 1 molecules in the micellar environment of nearly zero hydroxide ion concentration at >or=0.14 M C(16)E(20) and 1.0 x 10(-3) M NaOH while such observations could not be detected at


