(+)-MethysticinCAS# 495-85-2 |
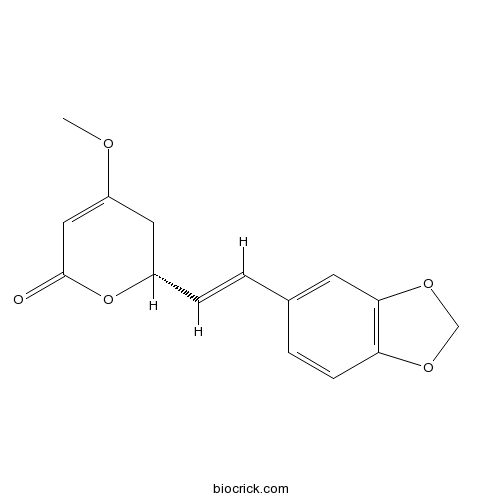
- Methysticin
Catalog No.:BCN2306
CAS No.:20697-20-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 495-85-2 | SDF | Download SDF |
| PubChem ID | 5281567 | Appearance | White powder |
| Formula | C15H14O5 | M.Wt | 274.27 |
| Type of Compound | Phenylpropanes | Storage | Desiccate at -20°C |
| Synonyms | Kavahin; Kavatin | ||
| Solubility | Freely soluble in dioxane and methanol; slightly soluble in water | ||
| Chemical Name | (2R)-2-[(E)-2-(1,3-benzodioxol-5-yl)ethenyl]-4-methoxy-2,3-dihydropyran-6-one | ||
| SMILES | COC1=CC(=O)OC(C1)C=CC2=CC3=C(C=C2)OCO3 | ||
| Standard InChIKey | GTEXBOVBADJOQH-FWEMWIAWSA-N | ||
| Standard InChI | InChI=1S/C15H14O5/c1-17-12-7-11(20-15(16)8-12)4-2-10-3-5-13-14(6-10)19-9-18-13/h2-6,8,11H,7,9H2,1H3/b4-2+/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

(+)-Methysticin Dilution Calculator

(+)-Methysticin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.646 mL | 18.2302 mL | 36.4604 mL | 72.9208 mL | 91.1511 mL |
| 5 mM | 0.7292 mL | 3.646 mL | 7.2921 mL | 14.5842 mL | 18.2302 mL |
| 10 mM | 0.3646 mL | 1.823 mL | 3.646 mL | 7.2921 mL | 9.1151 mL |
| 50 mM | 0.0729 mL | 0.3646 mL | 0.7292 mL | 1.4584 mL | 1.823 mL |
| 100 mM | 0.0365 mL | 0.1823 mL | 0.3646 mL | 0.7292 mL | 0.9115 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tigloyltropeine
Catalog No.:BCN1944
CAS No.:495-83-0
- Valtropine
Catalog No.:BCN1926
CAS No.:495-82-9
- Tropine isobutyrate
Catalog No.:BCN1923
CAS No.:495-80-7
- Desoxypeganine
Catalog No.:BCN8032
CAS No.:495-59-0
- Nodakenetin
Catalog No.:BCN5604
CAS No.:495-32-9
- Nodakenin
Catalog No.:BCN2378
CAS No.:495-31-8
- Ammijin
Catalog No.:BCN3617
CAS No.:495-30-7
- Auraptene
Catalog No.:BCN5603
CAS No.:495-02-3
- Ginsenoside Rk1
Catalog No.:BCN3552
CAS No.:494753-69-4
- TC-G 1001
Catalog No.:BCC6316
CAS No.:494191-73-0
- Nornicotine
Catalog No.:BCN8176
CAS No.:494-97-3
- Isoammodendrine
Catalog No.:BCN2146
CAS No.:494-15-5
- Org 25543 hydrochloride
Catalog No.:BCC6288
CAS No.:495076-64-7
- 11alpha,12alpha-Oxidotaraxerol palmitate
Catalog No.:BCN7129
CAS No.:495389-95-2
- Estradiol heptanoate
Catalog No.:BCC8961
CAS No.:4956-37-0
- Fenofibrate
Catalog No.:BCC4781
CAS No.:49562-28-9
- Benzofuran-2-carboxylic acid
Catalog No.:BCC8851
CAS No.:496-41-3
- Pyromeconic acid
Catalog No.:BCN7177
CAS No.:496-63-9
- Helicianeoide A
Catalog No.:BCN2486
CAS No.:496066-82-1
- Helicianeoide B
Catalog No.:BCN2487
CAS No.:496066-89-8
- Tetraethylenepentamine 5HCl
Catalog No.:BCC3867
CAS No.:4961-41-5
- Robustaflavone
Catalog No.:BCN8285
CAS No.:49620-13-5
- Angelicain
Catalog No.:BCN5605
CAS No.:49624-66-0
- Isomitraphylline
Catalog No.:BCN7800
CAS No.:4963-01-3
Kavalactone content and chemotype of kava beverages prepared from roots and rhizomes of Isa and Mahakea varieties and extraction efficiency of kavalactones using different solvents.[Pubmed:25694734]
J Food Sci Technol. 2015 Feb;52(2):1164-9.
The South Pacific islanders have consumed kava beverage for thousands of years. The quality of kava and kava beverage is evaluated through determination of the content of six major kavalactones including methysticin, dihydromethysticin, kavain, dihydrokavain, yangonin and desmethoxyyangonin. In this study, we determined contents of kavalactones in and chemotype of kava beverages prepared from roots and rhizomes of Isa and Mahakea varieties and extraction efficiency of five different solvents including hexane, acetone, methanol, ethanol and ethyl acetate. The six major kavalactones were detected in all kava beverages with these five solvents. Different solvents had different extraction efficiencies for kavalactones from the lyophilized kava preparations. The contents of kavalactones in the extracts with acetone, ethanol, and methanol did not differ significantly. Ethanol had the highest extraction efficiency for the six major kavalactones whereas hexane gave the lowest extraction efficiency.
Pacific island 'Awa (Kava) extracts, but not isolated kavalactones, promote proinflammatory responses in model mast cells.[Pubmed:22473598]
Phytother Res. 2012 Dec;26(12):1934-41.
Kava ('Awa) is a traditional water-based beverage in Pacific island communities, prepared from the ground root and stems of Piper methysticum. Kava use is associated with an ichthyotic dermatitis and delayed type hypersensitivity reactions. In the current study we collated preparative methodologies from cultural practitioners and recreational kava users in various Pacific communities. We standardized culturally informed aqueous extraction methods and prepared extracts that were subjected to basic physicochemical analysis. Mast cells exposed to these extracts displayed robust intracellular free calcium responses, and concomitant release of proinflammatory mediators. In contrast, mast cells were refractory to single or combinatorial stimulation with kavalactones, including methysticin, dihydromethysticin and kavain. Moreover, we reproduced a traditional modification of the kava preparation methodology, pre-mixing with the mucilage of Hibiscus tiliaceus, and observed its potentiating effect on the activity of aqueous extracts in mast cells. Taken together, these data indicate that water extractable active ingredients may play a role in the physiological and pathophysiological effects of kava, and suggests that mast cell activation may be a mechanistic component of kava-related skin inflammations.
Kavalactone metabolism in rat liver microsomes.[Pubmed:22807255]
Phytother Res. 2012 Jul;26(7):1057-61.
The specific CYP enzymes involved in kavalactone (KLT) metabolism and their kinetics have not been fully examined. This study used rat liver microsomes (RLM) to determine kavain (KA), methysticin (MTS) and desmethoxyyangonin (DMY) enzyme kinetic parameters, to elucidate the major CYP450 isoforms involved in KLT metabolism and to examine gender differences in KLT metabolism. Formation of the major KLT metabolites was first-order, consistent with classic enzyme kinetics. In both male and female RLM, clotrimazole (CLO) was the most potent inhibitor of KA and MTS metabolism. This suggests CYP3A1/3A23 (females) and CYP3A2 (males) are the main isoenzymes involved in the metabolism of these KLTs in rats, while the roles of CYP1A2, -2 C6, -2 C9, -2E1 and -3A4 are limited. Desmethoxyyangonin metabolism was equally inhibited by cimetidine (CIM) and CLO in females, and CIM and nortriptyline in males. This implies that DMY metabolism involves CYP2C6 and CYP2C11 in males, and CPY2C12 in females. CYP3A1/3A23 may also be involved in females.
Kavalactones, a novel class of protein glycation and lipid peroxidation inhibitors.[Pubmed:25098935]
Planta Med. 2014 Aug;80(12):1001-8.
Both advanced glycation endproducts and advanced lipoxidation endproducts are implicated in many age-related chronic diseases and in protein ageing. In this study, kawain, methysticin, and dihydromethysticin, all belonging to the group of kavalactones, were identified as advanced glycation endproduct inhibitors. With IC50 values of 43.5 +/- 1.2 microM and 45.0 +/- 1.3 microM for kawain and methysticin, respectively, the compounds inhibited the in vitro protein glycation significantly better than aminoguanidine (IC50 = 231.0 +/- 11.5 microM; p = 0.01), an established reference compound. Kawain and methysticin also inhibited the formation of dicarbonyl compounds, which are intermediates in the process of advanced glycation endproduct formation. Similarly, kawain and aminoguanidine prevented the formation of thiobarbituric reactive substances in both low-density lipoprotein and linoleic acid oxidation. Moreover, kawain and aminoguanidine prevented advanced glycation endproduct formation by chelating Fe(3+) and Cu(2+) two to three times better than aminoguanidine. Furthermore, kawain increased the mean life span of Caenorhabditis elegans exposed to high glucose. With glycation inhibiting, lipid peroxidation inhibiting, metal chelating properties, and life span extending ability, kavalactones show a high potential as advanced glycation endproducts and advanced lipoxidation endproduct inhibitors.
Phototoxicity of kava - formation of reactive oxygen species leading to lipid peroxidation and DNA damage.[Pubmed:23227797]
Am J Chin Med. 2012;40(6):1271-88.
Kava is one of the most widely sold herbal dietary supplements in the United States. It has been reported that, besides exhibiting hepatotoxicity, kava also possesses photosensitivity and induces dermopathy in humans. In this study, we determined that UVA irradiation of kava in the presence of a lipid, methyl linoleate, generated lipid peroxidation which was mediated by singlet oxygen generated during photoirradiation. The six major kavalactones(yangonin, 7,8-dihydrokawa in, kawain, 7,8-dihydromethysticin, methysticin, and 5,6-dehydrokawain) were also studied in parallel; only 5,6-dehydrokawain and yangonin-induced a low level of lipid peroxidation. UVA irradiation of kava in human HaCaT skin keratinocytes induced cytotoxicity which was mediated by oxidative stress, led to DNA strand cleavage, and produced 8-hydroxy-2'-deoxyguanosine (8-OHdG) adduct. Study by the electron spin resonance (ESR) method revealed that UVA irradiation of kava produced singlet oxygen and carbon-centered radicals. The overall results suggest that kava is photocytotoxic and photogenotoxic, both mediated by free radicals generated during photoirradiation.
Quantitative Determination of Lactones in Piper methysticum (Kava-Kava) by Supercritical Fluid Chromatography.[Pubmed:28095587]
Planta Med. 2017 Aug;83(12-13):1053-1057.
A fast and validated supercritical fluid chromatography method for the quantitative determination of major lactones in Piper methysticum, a plant used against nervous anxiety, stress, and restlessness, was developed. The baseline separation of dihydrokavain, demethoxyyangonin, kavain, yangonin, dihydromethysticin, and methysticin was possible in less than 4 min on an Aquity UPC(2) BEH 1.7 microm column, in combination with a mobile phase comprising CO2 and methanol with diethylamine. The column temperature had a great impact on the results because only at 70 degrees C could kavain and yangonin be fully resolved. With correlation coefficients above 0.998, recovery rates between 95.9 and 104.1 % as well as limit of detection values below 1.5 ng on-column, the procedure fulfilled all validation requirements and was well suited for the quantitative analysis of commercial products containing P. methysticum root powder and/or extract. All of them contained the target analytes, however, the absolute content of lactones was quite variable. Accordingly, depending on the product, the total daily intake of lactones varied from 56 to 312 mg. Concerning speed, selectivity, and environmental friendly operation, this supercritical fluid chromatography approach surpasses all previously reported ones.
Kavalactone metabolism in the isolated perfused rat liver.[Pubmed:22407838]
Phytother Res. 2012 Dec;26(12):1813-6.
Metabolic pathways for kavalactone metabolism in humans and rats have been identified, but more detailed description of the enzyme kinetics involved is lacking. The disposition profiles of three of the six major kavalactones (kavain, methysticin and desmethoxyyangonin) and their respective metabolites (p-hydroxykavain, m,p-dihydroxykavain and p-hydroxy-5,6-dehydrokavain) were examined in the perfusate and bile of the isolated perfused rat liver. The metabolism of the kavalactones is first-order in nature with similar decay half-lives. p-Hydroxykavain and m,p-dihydroxykavain were the only metabolites detected in the perfusate. Kavalactone biliary excretion was negligible.


