TC-G 1001CAS# 494191-73-0 |
- Nadifloxacin
Catalog No.:BCC4804
CAS No.:124858-35-1
- Calcipotriol monohydrate
Catalog No.:BCC1445
CAS No.:147657-22-5
- Halobetasol Propionate
Catalog No.:BCC4664
CAS No.:66852-54-8
- Dihydroartemisinin
Catalog No.:BCN6264
CAS No.:71939-50-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 494191-73-0 | SDF | Download SDF |
| PubChem ID | 5766343 | Appearance | Powder |
| Formula | C17H11FN2O3S | M.Wt | 342.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in DMSO | ||
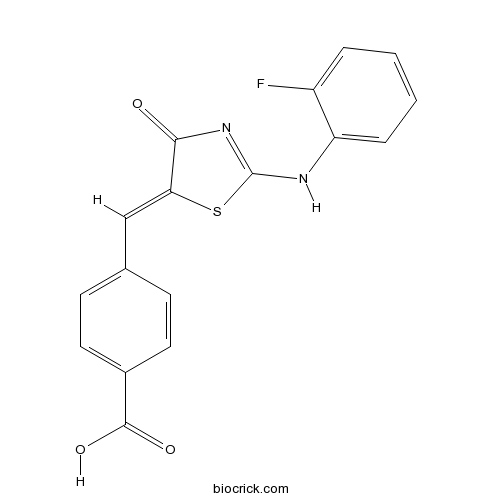
| Chemical Name | 4-[(Z)-[2-(2-fluoroanilino)-4-oxo-1,3-thiazol-5-ylidene]methyl]benzoic acid | ||
| SMILES | C1=CC=C(C(=C1)NC2=NC(=O)C(=CC3=CC=C(C=C3)C(=O)O)S2)F | ||
| Standard InChIKey | SYCKPHBALHXMIR-ZROIWOOFSA-N | ||
| Standard InChI | InChI=1S/C17H11FN2O3S/c18-12-3-1-2-4-13(12)19-17-20-15(21)14(24-17)9-10-5-7-11(8-6-10)16(22)23/h1-9H,(H,22,23)(H,19,20,21)/b14-9- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GPR35 agonist (pEC50 values are 7.59 and 8.36 for β-arrestin and Gαq-i5 Ca2+ assays respectively). Displays 1000-fold greater potency at human GPR35 receptors relative to mouse and rat GPR35 orthologs in an IP1 accumulation assay. More potent than zaprinast. |

TC-G 1001 Dilution Calculator

TC-G 1001 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9211 mL | 14.6054 mL | 29.2107 mL | 58.4215 mL | 73.0268 mL |
| 5 mM | 0.5842 mL | 2.9211 mL | 5.8421 mL | 11.6843 mL | 14.6054 mL |
| 10 mM | 0.2921 mL | 1.4605 mL | 2.9211 mL | 5.8421 mL | 7.3027 mL |
| 50 mM | 0.0584 mL | 0.2921 mL | 0.5842 mL | 1.1684 mL | 1.4605 mL |
| 100 mM | 0.0292 mL | 0.1461 mL | 0.2921 mL | 0.5842 mL | 0.7303 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nornicotine
Catalog No.:BCN8176
CAS No.:494-97-3
- Isoammodendrine
Catalog No.:BCN2146
CAS No.:494-15-5
- Nifuratel
Catalog No.:BCC1800
CAS No.:4936-47-4
- Methyl L-pyroglutamate
Catalog No.:BCN7060
CAS No.:4931-66-2
- Savinin
Catalog No.:BCN5602
CAS No.:493-95-8
- α-D-Glucose
Catalog No.:BCC9197
CAS No.:492-62-6
- Kynurenic acid
Catalog No.:BCN2228
CAS No.:492-27-3
- Butin
Catalog No.:BCN4630
CAS No.:492-14-8
- Plathymenin
Catalog No.:BCN6810
CAS No.:492-12-6
- (+)-Sparteine
Catalog No.:BCC9249
CAS No.:492-08-0
- Thermopsidine
Catalog No.:BCN7923
CAS No.:492-02-4
- TCS PIM-1 1
Catalog No.:BCC2447
CAS No.:491871-58-0
- Ginsenoside Rk1
Catalog No.:BCN3552
CAS No.:494753-69-4
- Auraptene
Catalog No.:BCN5603
CAS No.:495-02-3
- Ammijin
Catalog No.:BCN3617
CAS No.:495-30-7
- Nodakenin
Catalog No.:BCN2378
CAS No.:495-31-8
- Nodakenetin
Catalog No.:BCN5604
CAS No.:495-32-9
- Desoxypeganine
Catalog No.:BCN8032
CAS No.:495-59-0
- Tropine isobutyrate
Catalog No.:BCN1923
CAS No.:495-80-7
- Valtropine
Catalog No.:BCN1926
CAS No.:495-82-9
- Tigloyltropeine
Catalog No.:BCN1944
CAS No.:495-83-0
- (+)-Methysticin
Catalog No.:BCN8429
CAS No.:495-85-2
- Org 25543 hydrochloride
Catalog No.:BCC6288
CAS No.:495076-64-7
- 11alpha,12alpha-Oxidotaraxerol palmitate
Catalog No.:BCN7129
CAS No.:495389-95-2
High-throughput identification and characterization of novel, species-selective GPR35 agonists.[Pubmed:23262279]
J Pharmacol Exp Ther. 2013 Mar;344(3):568-78.
Drugs targeting the orphan receptor GPR35 have potential therapeutic application in a number of disease areas, including inflammation, metabolic disorders, nociception, and cardiovascular disease. Currently available surrogate GPR35 agonists identified from pharmacologically relevant compound libraries have limited utility due to the likelihood of off-target effects in vitro and in vivo and the variable potency that such ligands exhibit across species. We sought to identify and characterize novel GPR35 agonists to facilitate studies aimed at defining the physiologic role of GPR35. PathHunter beta-arrestin recruitment technology was validated as a human GPR35 screening assay, and a high-throughput screen of 100,000 diverse low molecular weight compounds was conducted. Confirmed GPR35 agonists from five distinct chemotypes were selected for detailed characterization using both beta-arrestin recruitment and G protein-dependent assays and each of the human, mouse, and rat GPR35 orthologs. These studies identified 4-{(Z)-[(2Z)-2-(2-fluorobenzylidene)-4-oxo-1,3-thiazolidin-5-ylidene]methyl}benzo ic acid (compound 1) as the highest potency full agonist of human GPR35 yet described. As with certain other GPR35 agonists, compound 1 was markedly selective for human GPR35, but displayed elements of signal bias between beta-arrestin-2 and G protein-dependent assays. Compound 1 also displayed competitive behavior when assessed against the human GPR35 antagonist, ML-145 (2-hydroxy-4-[4-(5Z)-5-[(E)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-sulfanylid ene-1,3-thiazolidin-3-yl]butanoylamino]benzoic acid). Of the other chemotypes studied, compounds 2 and 3 were selective for the human receptor, but compounds 4 and 5 demonstrated similar activity at human, rat, and mouse GPR35 orthologs. Further characterization of these compounds and related analogs is likely to facilitate a better understanding of GPR35 in health and disease.


