Methylpheophorbide ACAS# 5594-30-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5594-30-9 | SDF | Download SDF |
| PubChem ID | 135407639 | Appearance | Powder |
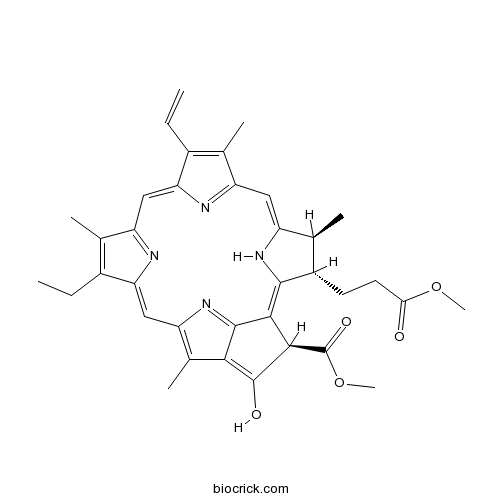
| Formula | C36H38N4O5 | M.Wt | 606.71 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Methyl pheophorbide a | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl (3R,21S,22S)-16-ethenyl-11-ethyl-4-hydroxy-22-(3-methoxy-3-oxopropyl)-12,17,21,26-tetramethyl-7,23,24,25-tetrazahexacyclo[18.2.1.15,8.110,13.115,18.02,6]hexacosa-1,4,6,8(26),9,11,13(25),14,16,18(24),19-undecaene-3-carboxylate | ||
| SMILES | CCC1=C(C2=NC1=CC3=C(C4=C(C(C(=C5C(C(C(=CC6=NC(=C2)C(=C6C)C=C)N5)C)CCC(=O)OC)C4=N3)C(=O)OC)O)C)C | ||
| Standard InChIKey | HUXSMOZWPXDRTN-SDHKEVEOSA-N | ||
| Standard InChI | InChI=1S/C36H38N4O5/c1-9-20-16(3)23-13-25-18(5)22(11-12-29(41)44-7)33(39-25)31-32(36(43)45-8)35(42)30-19(6)26(40-34(30)31)15-28-21(10-2)17(4)24(38-28)14-27(20)37-23/h9,13-15,18,22,32,39,42H,1,10-12H2,2-8H3/t18-,22-,32+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Methylphophorbide A shows antioxidative and anticarcinogenic activities. |

Methylpheophorbide A Dilution Calculator

Methylpheophorbide A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6482 mL | 8.2412 mL | 16.4823 mL | 32.9647 mL | 41.2058 mL |
| 5 mM | 0.3296 mL | 1.6482 mL | 3.2965 mL | 6.5929 mL | 8.2412 mL |
| 10 mM | 0.1648 mL | 0.8241 mL | 1.6482 mL | 3.2965 mL | 4.1206 mL |
| 50 mM | 0.033 mL | 0.1648 mL | 0.3296 mL | 0.6593 mL | 0.8241 mL |
| 100 mM | 0.0165 mL | 0.0824 mL | 0.1648 mL | 0.3296 mL | 0.4121 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Betamethasone 17,21-dipropionate
Catalog No.:BCC8875
CAS No.:5593-20-4
- Pennogenin 3-O-beta-chacotrioside
Catalog No.:BCN6707
CAS No.:55916-52-4
- Polyphyllin VI
Catalog No.:BCN1053
CAS No.:55916-51-3
- Cucurbitacin R
Catalog No.:BCN7877
CAS No.:55903-92-9
- Friedelin
Catalog No.:BCN5747
CAS No.:559-74-0
- beta-Amyrin
Catalog No.:BCN5746
CAS No.:559-70-6
- Morolic acid
Catalog No.:BCN7475
CAS No.:559-68-2
- 6-Hydroxysugiol
Catalog No.:BCN3154
CAS No.:55898-07-2
- Boc-D-Lys(Z)-OH
Catalog No.:BCC3423
CAS No.:55878-47-2
- 6-beta-Hydroxyhyoscyamine
Catalog No.:BCN1915
CAS No.:55869-99-3
- VER-49009
Catalog No.:BCC5297
CAS No.:558640-51-0
- 1-Methoxymethyl-beta-carboline
Catalog No.:BCN7911
CAS No.:55854-60-9
- Pseudohypericin
Catalog No.:BCN6348
CAS No.:55954-61-5
- ARC 239 dihydrochloride
Catalog No.:BCC6851
CAS No.:55974-42-0
- Nitazoxanide
Catalog No.:BCC3824
CAS No.:55981-09-4
- Methylthiouracil
Catalog No.:BCC4800
CAS No.:56-04-2
- 2,4-Diamino-6-hydroxypyrimidine
Catalog No.:BCC6658
CAS No.:56-06-4
- 4-Aminobutanoic acid
Catalog No.:BCN2187
CAS No.:56-12-2
- Cystamine dihydrochloride
Catalog No.:BCC6344
CAS No.:56-17-7
- Cantharidin
Catalog No.:BCN1280
CAS No.:56-25-7
- Tetraethylammonium chloride
Catalog No.:BCC7554
CAS No.:56-34-8
- H-Gly-OH
Catalog No.:BCC2946
CAS No.:56-40-6
- H-Ala-OH
Catalog No.:BCC3190
CAS No.:56-41-7
- H-Ser-OH
Catalog No.:BCC3028
CAS No.:56-45-1
Antioxidative and anticarcinogenic activities of methylpheophorbide a, isolated from wheat grass (Triticum aestivum Linn.).[Pubmed:25782530]
Nat Prod Res. 2016;30(4):474-7.
Methylphophorbide a (MPa) has been isolated from the ethanol extract of the wheat grass plant. Its antioxidative efficacy is evaluated by hydroxyl radical scavenging activities and reducing capacity which are significantly up regulated in comparison with aqueous extract of the plant. The compound shows iron-binding capacity where the Fe(2+) binds with MPa by two types of binding patterns with dissociation constants 157.17 and 27.89. It has antioxidative and cytotoxic effects on HeLa and Hep G2 cells. The cancerous cell survivability decreases with increasing concentration of MPa. These findings have provided evidence for the traditional use of the wheat grass plant in the treatment of cancers, oxidative stress and iron overloaded disorders.
Semi-synthesis and PDT activities of a new amphiphilic chlorin derivative.[Pubmed:27769913]
Photodiagnosis Photodyn Ther. 2017 Mar;17:39-47.
An amphiphilic chlorin derivative (CHL-T) was prepared from Methylpheophorbide A (CHL) and 2-Amino-2-(hydroxymethyl)-1,3-propanediol (TRISMA((R))). The new chlorin was compared to other dyes (CHL and Hypericin) in relation to photophysical and photobiological activities in tumor and non-tumor cell lines. Cytotoxicity and cell death target were determined to evaluate the CHL-T efficiency, comparing to the precursor CHL and to the well-known dye hypericin (HY). All of the studied compounds exhibited absorption bands in the therapeutic window and presented a small fluorescence quantum yield compared to the reference dye (rhodamine B). CHL-T was about three times more efficient on singlet oxygen generation than the others photosensitizers. The lipophilicity order of the photosensitizers was CHL>HY>CHL-T. The tumoral HeLa cells presented improved accumulation for CHL and CHL-T compared to HY. The phototoxicity presented by the CHL-T was about ten times higher than by CHL, as demonstrated by the MTT assay. CHL-T showed more cytotoxicity to tumoral cell, comparing to non-tumoral cell in short incubation time. The cell death rises proportionally with increasing PSs concentrations, mainly by necrosis. These findings suggest that CHL-T is a potential new photosensitizer for PDT.


