1-Methoxymethyl-beta-carbolineCAS# 55854-60-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

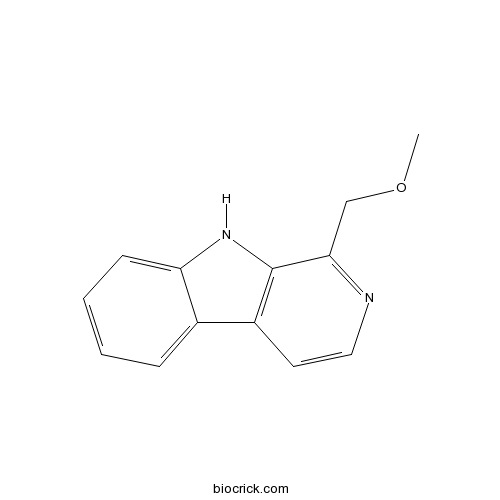
| Cas No. | 55854-60-9 | SDF | Download SDF |
| PubChem ID | 5382686 | Appearance | Powder |
| Formula | C13H12N2O | M.Wt | 212.25 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-(methoxymethyl)-9H-pyrido[3,4-b]indole | ||
| SMILES | COCC1=NC=CC2=C1NC3=CC=CC=C23 | ||
| Standard InChIKey | OELXEULZDKMFCS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H12N2O/c1-16-8-12-13-10(6-7-14-12)9-4-2-3-5-11(9)15-13/h2-7,15H,8H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1-Methoxymethyl-beta-carboline is a natural product from Picrasma javanica. |
| Structure Identification | Biol Pharm Bull. 1993 Feb;16(2):103-6.Fluorometric determination of L-tryptophan with methoxyacetaldehyde.[Pubmed: 8364442]
|

1-Methoxymethyl-beta-carboline Dilution Calculator

1-Methoxymethyl-beta-carboline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.7114 mL | 23.5571 mL | 47.1143 mL | 94.2285 mL | 117.7856 mL |
| 5 mM | 0.9423 mL | 4.7114 mL | 9.4229 mL | 18.8457 mL | 23.5571 mL |
| 10 mM | 0.4711 mL | 2.3557 mL | 4.7114 mL | 9.4229 mL | 11.7786 mL |
| 50 mM | 0.0942 mL | 0.4711 mL | 0.9423 mL | 1.8846 mL | 2.3557 mL |
| 100 mM | 0.0471 mL | 0.2356 mL | 0.4711 mL | 0.9423 mL | 1.1779 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AMD-070
Catalog No.:BCC1357
CAS No.:558447-26-0
- Halofuginone
Catalog No.:BCN8519
CAS No.:55837-20-2
- Columbianetin beta-D-glucopyranoside
Catalog No.:BCN8222
CAS No.:55836-35-6
- Betahistine 2HCl
Catalog No.:BCC4525
CAS No.:5579-84-0
- Boc-D-Allo-Ile-OH
Catalog No.:BCC2603
CAS No.:55780-90-0
- Sanguinarine chloride
Catalog No.:BCC6481
CAS No.:5578-73-4
- Sunitinib
Catalog No.:BCC4064
CAS No.:557795-19-4
- WZ811
Catalog No.:BCC4448
CAS No.:55778-02-4
- Crocin II
Catalog No.:BCN1027
CAS No.:55750-84-0
- Litorin
Catalog No.:BCC5846
CAS No.:55749-97-8
- Oxoglaucine
Catalog No.:BCN2650
CAS No.:5574-24-3
- 3-Deoxo-1Beta-methoxyjioglutolide
Catalog No.:BCN7034
CAS No.:55732-36-0
- VER-49009
Catalog No.:BCC5297
CAS No.:558640-51-0
- 6-beta-Hydroxyhyoscyamine
Catalog No.:BCN1915
CAS No.:55869-99-3
- Boc-D-Lys(Z)-OH
Catalog No.:BCC3423
CAS No.:55878-47-2
- 6-Hydroxysugiol
Catalog No.:BCN3154
CAS No.:55898-07-2
- Morolic acid
Catalog No.:BCN7475
CAS No.:559-68-2
- beta-Amyrin
Catalog No.:BCN5746
CAS No.:559-70-6
- Friedelin
Catalog No.:BCN5747
CAS No.:559-74-0
- Cucurbitacin R
Catalog No.:BCN7877
CAS No.:55903-92-9
- Polyphyllin VI
Catalog No.:BCN1053
CAS No.:55916-51-3
- Pennogenin 3-O-beta-chacotrioside
Catalog No.:BCN6707
CAS No.:55916-52-4
- Betamethasone 17,21-dipropionate
Catalog No.:BCC8875
CAS No.:5593-20-4
- Methylpheophorbide A
Catalog No.:BCN7998
CAS No.:5594-30-9
Fluorometric determination of L-tryptophan with methoxyacetaldehyde.[Pubmed:8364442]
Biol Pharm Bull. 1993 Feb;16(2):103-6.
It was observed in our laboratory that methoxyacetaldehyde induced strong fluorescence with L-tryptophan in the presence of NaNO2 at pH 2.75. The reaction mixture was heated at 80 degrees C for 15 min and the fluorescence was measured at the excitation wavelength, 253 nm and the emission, 450 nm. Amino acids other than L-tryptophan did not produce fluorescence under the conditions employed. A good linear working curve was observed between 25 pmol/50 microliters and 500 pmol/50 microliters of L-tryptophan. The limit of determination for L-tryptophan was 10 pmol/50 microliters. The coefficient of variation of the measurements (n = 5) was 1.5% for 250 pmol/50 microliters of L-tryptophan. The proposed method was successfully applied to the analyses of tryptophan in a pooled human serum. Total tryptophan was determined after deproteinization with trichloroacetic acid, and free tryptophan was determined using the ultrafiltrate of a pooled human serum. Analytical recoveries of total and free tryptophan were 98.8% and 99.1%, respectively. The fluorescent products of L-tryptophan and methoxyacetaldehyde were characterized as beta-carboline and 1-Methoxymethyl-beta-carboline by the use of liquid chromatography-mass spectrometry (LC-MS) and HPLC.


