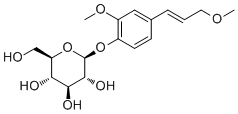
MethylconiferinCAS# 883150-46-7 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 883150-46-7 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Powder |
| Formula | C17H24O8 | M.Wt | 356.41 |
| Type of Compound | Phenylpropanoid | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Methylconiferin Dilution Calculator

Methylconiferin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8058 mL | 14.0288 mL | 28.0576 mL | 56.1151 mL | 70.1439 mL |
| 5 mM | 0.5612 mL | 2.8058 mL | 5.6115 mL | 11.223 mL | 14.0288 mL |
| 10 mM | 0.2806 mL | 1.4029 mL | 2.8058 mL | 5.6115 mL | 7.0144 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5612 mL | 0.7014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Iristectorin B
Catalog No.:BCX0505
CAS No.:94396-09-5
- Vavain
Catalog No.:BCX0504
CAS No.:199996-77-5
- 6-Demethoxyirigenin
Catalog No.:BCX0503
CAS No.:1348833-10-2
- Oxyphyllenodiol A
Catalog No.:BCX0502
CAS No.:363610-30-4
- Teuhetenone A
Catalog No.:BCX0501
CAS No.:152481-80-6
- Isocrenatoside
Catalog No.:BCX0500
CAS No.:221895-09-6
- Erythrinin E
Catalog No.:BCX0499
CAS No.:2731101-51-0
- Crenatoside
Catalog No.:BCX0498
CAS No.:61276-16-2
- Curdionolide B
Catalog No.:BCX0497
CAS No.:1190225-68-3
- 7,4'-Di-O-methylaromadendrin
Catalog No.:BCX0496
CAS No.:41515-76-8
- Sanggenol C
Catalog No.:BCX0495
CAS No.:174423-32-6
- Rugulolide D
Catalog No.:BCX0494
CAS No.:3002032-69-8
- 1β-Hydroxy-8α-methoxyeremophila-7(11),9-dien-12,8β-olide
Catalog No.:BCX0507
CAS No.:849700-45-4
- (S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-phenylheptan-3-one
Catalog No.:BCX0508
CAS No.:1220110-76-8
- Moracin N
Catalog No.:BCX0509
CAS No.:135248-05-4
- D-Mannosamine hydrochloride
Catalog No.:BCX0510
CAS No.:5505-63-5
- D-Galactosamine hydrochloride
Catalog No.:BCX0511
CAS No.:1772-03-8
- N-acetyl-D-galactosamine
Catalog No.:BCX0512
CAS No.:1811-31-0
- N-Acetyl-D-mannosamine
Catalog No.:BCX0513
CAS No.:7772-94-3
- Xanthomicrol
Catalog No.:BCX0514
CAS No.:16545-23-6
- 5,7-Dihydroxy-3,8,3',4'-tetramethoxyflavone
Catalog No.:BCX0515
CAS No.:42923-42-2
- 5,4'-Dihydroxy-6,7,8,3'-tetramethoxyflavone
Catalog No.:BCX0516
CAS No.:16520-78-8
- Tetrahydroauroglaucin
Catalog No.:BCX0517
CAS No.:40434-07-9
- Sanggenon F
Catalog No.:BCX0518
CAS No.:85889-03-8
Effect of Cedrus deodara extract on the physiochemical and sensory properties of salted meat and its action mechanism.[Pubmed:34147039]
J Food Sci. 2021 Jul;86(7):2910-2923.
The effect of pine needle extract from Cedrus deodara (PNE) on the quality of salted meat was reported, and its action mechanism was further investigated. With the treatment of PNE, the physicochemical properties of salted meat were improved. The peroxide value decreased from 16.18 to 6.78 mmol O(2) /kg, while the thiobarbituric acid value decreased from 0.79 to 0.40 mg MDA/kg. Moreover, the salted meat with PNE also had the better texture, color, and volatile compositions. The 0.2% PNE group showed the highest DeltaE value (63.16 +/- 0.56), hardness (813.5 +/- 48.7 g), and volatility (45.86 +/- 0.39), while the control group showed the lowest DeltaE value (43.92 +/- 2.13), hardness (515.8 +/- 17.3 g) and volatility (29.97 +/- 0.56). In addition, with the analysis of fluorescence and circular dichroism spectroscopy, the spatial structures of myofibrillar protein (MP) in salted meat were obviously changed by PNE. Meanwhile, Methylconiferin, 1-O-feruloyl-beta-D-glucose, nortrachelogenin, secoxyloganin, 1-O-(4-coumaroyl)-beta-D-glucose and pelargonidin-3-O-glucoside were identified from PNE. Furthermore, according to the analysis of molecular docking, hydrogen bond, hydrophobic force, and electrostatic force were obtained as the main molecular forces between MP and the phenolic compounds of PNE, while arginine, glutamic acid, and glycine residues were the main binding sites. All results suggested that PNE might be a potential candidate to improve the quality of salted meat in the food industry. PRACTICAL APPLICATION: The quality deterioration of meat may not only affect its further processing and consumption but also may lead to some food safety problems. In present study, PNE exhibited the fine capability to inhibit the oxidation of meat, while it could ameliorate the texture, color, and physicochemical properties of meat due to its tightly interaction with myofibrillar protein. All result suggested that PNE could be potentially utilized to improve the quality of meat in food industry.
Anti-inflammatory effects of chemical components from Ginkgo biloba L. male flowers on lipopolysaccharide-stimulated RAW264.7 macrophages.[Pubmed:30693991]
Phytother Res. 2019 Apr;33(4):989-997.
Ginkgo biloba L., well known as living fossil, have various pharmacological activities. Eighteen compounds were isolated from Ginkgo male flowers including a novel matsutake alcohol glycoside, Ginkgoside A (1), and 17 known compounds-calaliukiuenoside (2), benzylalcohol O-alpha-l-arabinopyranosyl-(1 --> 6)-beta-d-glucopyranoside (3), amentoflavone (4), sciadopitysin (5), bilobetin (6), isoginkgetin (7), olivil 4-O-beta-d-glucopyranoside (8), dihydrodehydrodiconiferyl alcohol-4-O-beta-d-glucoside (9), (+)-cyclo-olivil-6-O-beta-d-glucopyranoside (10), (-)-isolariciresinol 4-O-beta-d-glucopyranoside (11), coniferin (12), trans-cinnamic acid-4-O-beta-d-glucopyranoside (13), p-coumaryl alchol glucoside (14), stroside B (15), Methylconiferin (16), cis-p-coumaric acid 4-O-beta-d-glucopyranoside (17), and cis-coniferin (18). Thirteen of these compounds had not previously found in Ginkgo. All extractive fractions and isolated compounds were evaluated for their anti-inflammatory ability in the lipopolysaccharide-induced RAW264.7 macrophages. The ethanol extract of Ginkgo flowers and the chloroform and ethyl acetate fractions can significantly decrease nitric oxide (NO), interleukin-6 (IL-6), and prostaglandin E(2) (PGE(2) ) production at 100 mug/ml. The most effective compounds, bilobetin (6) and isoginkgetin (7), elevated the NO inhibition ratios to 80.19% and 82.37% at 50 muM, respectively. They also exhibited significant dose-dependent inhibitory effects on tumor necrosis factor-alpha, IL-6, PGE(2) , inducible NO synthase mRNA, and cyclooxygenase-2 mRNA levels. So they can be promising candidates for the development of new anti-inflammatory agents.
Chemical constituents of Arisaema franchetianum tubers.[Pubmed:23106482]
J Asian Nat Prod Res. 2013;15(1):71-7.
A novel pyrrolidine alkaloid, (2R*,3S*,5S*)-N,2-dimethyl-3-hydroxy-5-(10-phenyldecyl)pyrrolidine (1), and 17 known compounds were isolated from Arisaema franchetianum Engl. (Araceae) tubers. The 17 compounds were bergenin (2), emodin (3), caffeic acid (4), nobiletin (5), 3-O-beta-d-galactopyranosyl-hederagenin 28-O-beta-d-xylopyranosyl(1 --> 6)-beta-d-galactopyranosyl ester (6), coniferin (7), qingyangshengenin (8), Methylconiferin (9), syringaresinol 4'-O-beta-d-glucopyranoside (10), gagaminine (11), perlolyrine (12), (S)-1-(1'-hydroxyethyl)-beta-carboline (13), 1-(beta-carboline-1-yl)-3,4,5-trihydroxy-1-pentanone (14), 1-methoxycarbonyl-beta-carboline (15), indolo[2,3-alpha]carbazole (16), 4-hydroxycinnamic acid methyl ester (17), and methyl 4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethyl] ferulate (18). The inhibitory activities of compound 1 and its N-methyl derivative (1a) against porcine respiratory and reproductive syndrome virus (PRRSV), human leukemic K562 cells, and human breast cancer MCF-7 cells were evaluated. Compounds 1 [50% inhibited concentration (IC(50)) = 12.5 +/- 0.6 muM] and 1a (IC(50) = 15.7 +/- 0.9 muM) were cytotoxic against K562 cells. Compound 1a also had a weak effect on PRRSV with an IC(50) value of 31.9 +/- 6.0 muM [selectivity index (SI) = 18.7].
[Study on the chemical constituents from pine needles of Cedrus deodara].[Pubmed:20575413]
Zhong Yao Cai. 2010 Feb;33(2):215-8.
OBJECTIVE: To study the chemical constituents from pine needles of Cedrus deodara. METHODS: Chemical constituents were isolated by silica gel and Sephadex LH-20 column chromatography. The structures of the isolated compounds were elucidated through spectroscopic analysis. RESULTS: The compounds were identified as 9-hydroxy-dodecanoic acid (I), ethyl laurate (II), ethyl stearate (III), 3beta-hydroxy-oleanolic acid methyl ester (IV), beta-sitosterol (V), shikimic acid (VI), Methylconiferin (VII), ferulic acid beta-glucoside (VIII). CONCLUSION: Compounds I-IV, VI-VIII are isolated and identified from this genus for the first time, compound V is isolated from pine needles of this genus for the first time.


