IsocrenatosideCAS# 221895-09-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

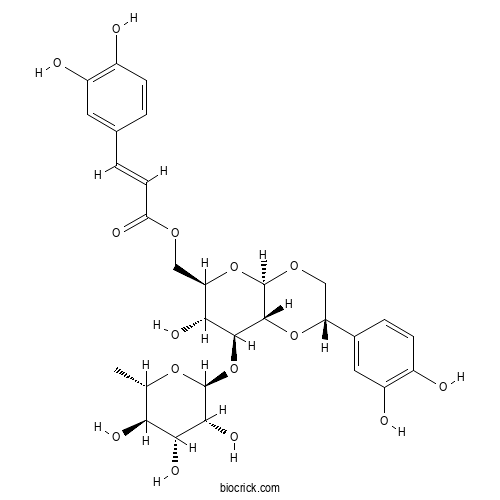
| Cas No. | 221895-09-6 | SDF | Download SDF |
| PubChem ID | 44559534 | Appearance | Powder |
| Formula | C29H34O15 | M.Wt | 622.6 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2S,4aR,6R,7R,8S,8aR)-2-(3,4-dihydroxyphenyl)-7-hydroxy-8-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-3,4a,6,7,8,8a-hexahydro-2H-pyrano[2,3-b][1,4]dioxin-6-yl]methyl (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(OC3C2OC(CO3)C4=CC(=C(C=C4)O)O)COC(=O)C=CC5=CC(=C(C=C5)O)O)O)O)O)O | ||
| Standard InChIKey | FYNJOHBQQZWZTB-WLLRULDYSA-N | ||
| Standard InChI | InChI=1S/C29H34O15/c1-12-22(35)24(37)25(38)28(41-12)44-26-23(36)20(11-39-21(34)7-3-13-2-5-15(30)17(32)8-13)43-29-27(26)42-19(10-40-29)14-4-6-16(31)18(33)9-14/h2-9,12,19-20,22-33,35-38H,10-11H2,1H3/b7-3+/t12-,19+,20+,22-,23+,24+,25+,26-,27+,28-,29+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isocrenatoside Dilution Calculator

Isocrenatoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6062 mL | 8.0308 mL | 16.0617 mL | 32.1234 mL | 40.1542 mL |
| 5 mM | 0.3212 mL | 1.6062 mL | 3.2123 mL | 6.4247 mL | 8.0308 mL |
| 10 mM | 0.1606 mL | 0.8031 mL | 1.6062 mL | 3.2123 mL | 4.0154 mL |
| 50 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6425 mL | 0.8031 mL |
| 100 mM | 0.0161 mL | 0.0803 mL | 0.1606 mL | 0.3212 mL | 0.4015 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Erythrinin E
Catalog No.:BCX0499
CAS No.:2731101-51-0
- Crenatoside
Catalog No.:BCX0498
CAS No.:61276-16-2
- Curdionolide B
Catalog No.:BCX0497
CAS No.:1190225-68-3
- 7,4'-Di-O-methylaromadendrin
Catalog No.:BCX0496
CAS No.:41515-76-8
- Sanggenol C
Catalog No.:BCX0495
CAS No.:174423-32-6
- Rugulolide D
Catalog No.:BCX0494
CAS No.:3002032-69-8
- Sanggenon E
Catalog No.:BCX0493
CAS No.:81381-69-3
- 4-Hydroxy-4-methylcyclohex-2-en-1-one
Catalog No.:BCX0492
CAS No.:70150-56-0
- 7-epi-α-Cyperone
Catalog No.:BCX0491
CAS No.:547-26-2
- Ethyl 2,4-dihydroxybenzoate
Catalog No.:BCX0490
CAS No.:4143-00-4
- 2,4-Dihydroxybezaldehyde
Catalog No.:BCX0489
CAS No.:95-01-2
- Rugulolide B
Catalog No.:BCX0488
CAS No.:3002032-71-2
- Teuhetenone A
Catalog No.:BCX0501
CAS No.:152481-80-6
- Oxyphyllenodiol A
Catalog No.:BCX0502
CAS No.:363610-30-4
- 6-Demethoxyirigenin
Catalog No.:BCX0503
CAS No.:1348833-10-2
- Vavain
Catalog No.:BCX0504
CAS No.:199996-77-5
- Iristectorin B
Catalog No.:BCX0505
CAS No.:94396-09-5
- Methylconiferin
Catalog No.:BCX0506
CAS No.:883150-46-7
- 1β-Hydroxy-8α-methoxyeremophila-7(11),9-dien-12,8β-olide
Catalog No.:BCX0507
CAS No.:849700-45-4
- (S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-phenylheptan-3-one
Catalog No.:BCX0508
CAS No.:1220110-76-8
- Moracin N
Catalog No.:BCX0509
CAS No.:135248-05-4
- D-Mannosamine hydrochloride
Catalog No.:BCX0510
CAS No.:5505-63-5
- D-Galactosamine hydrochloride
Catalog No.:BCX0511
CAS No.:1772-03-8
- N-acetyl-D-galactosamine
Catalog No.:BCX0512
CAS No.:1811-31-0
A new phenylethanoid glycoside from Orobanche cernua Loefling.[Pubmed:26358786]
Nat Prod Res. 2016;30(8):948-53.
A novel phenylethanoid glycoside, 3'-O-methyl Isocrenatoside (1), along with two known compounds, methyl caffeate (2) and protocatechuic aldehyde (3), were isolated from the fresh whole plant of Orobanche cernua Loefling. All the isolated compounds (1-3) were elucidated on the basis of spectroscopic analysis including IR, MS and NMR data. The cytotoxic activities of these compounds were evaluated. Results showed that 3'-O-methyl Isocrenatoside (1) and methyl caffeate (2) exhibited significant cytotoxicity, with IC50 values of 71.89, 36.97 mug/mL and 32.32, 34.58 mug/mL against the B16F10 murine melanoma and Lewis lung carcinoma cell lines, respectively.
[Nonvolatile chemical constituents from Pogostemon cablin].[Pubmed:21246823]
Zhongguo Zhong Yao Za Zhi. 2010 Oct;35(20):2704-7.
OBJECTIVE: To investigate the nonvolatile chemical constituents from the ethanol extract of the stems of Pogostemon cablin. METHOD: The constituents were isolated and purified by repeated column chromatography on silica gel and Sephadex LH-20. Their structures were identified by physicochemical properties and spectroscopic analysis. RESULT: Twelve compounds were isolated and identified as tilianin (1), diosmetin-7-O-beta-D-glucopyranoside (2), 3"-O-methylcrenatoside (3), uracil (4), soya-cerebroside I and II (5), agastachoside (6), apigenin-7-O-(3", 6"-di-(E) -p-coumaroyl) -beta-D-galactopyranoside (7), 5-hydroxy-3, 3', 4', 7- tetramethoxy flavone (8), 4', 5-dihydroxy-3, 3', 7-trimethoxyflavone (9), acacetin (10), crenatoside (11), Isocrenatoside (12). CONCLUSION: Compounds 1, 2, 4-7, 10 were isolated from the genus Pogostemon for the first time.
[Chemical constituents from involatile moiety of Pogostemon cablin].[Pubmed:19459301]
Zhongguo Zhong Yao Za Zhi. 2009 Feb;34(4):410-3.
OBJECTIVE: To study the chemical constituents of involatile moiety of Pogostemon cablin. METHOD: Compounds were isolated and purified by repeated column chromatography, and their structures were elucidated by spectroscopic analysis. RESULT: Nine compounds have been isolated and identified: epifriedelinol (1), 5-hydroxymethol-2-furfural (2), succinic acid (3), beta-sitosterol (4), daucosterol (5), crenatoside (6), 3'''-O-methylcrenatoside (7), Isocrenatoside (8), and apigenin-7-O-beta-D-(6"-p-coumaryl)-glucoside (9). CONCLUSION: Compounds 2, 3, 6-8 were isolated from Pogostemon genus for the first time.
Three cyclooctapeptides and one glycoside from Microtoena prainiana.[Pubmed:15217277]
J Nat Prod. 2004 Jun;67(6):978-82.
Three new cyclic octapeptides, microtoenins A-C (1-3), and a new glycoside, 3'''-O-methylcrenatoside (4), along with several known compounds, were isolated from the ethanolic extract of the stems of Microtoena prainiana. Their structures were determined by spectral and chemical evidence. At a concentration of 0.01 mg/mL, 3'''-O-methylcrenatoside (4), crenatoside (5), and Isocrenatoside (6) inhibited angiotensin converting enzyme (ACE) activity by more than 30%.
Iridoid glycosides from Globularia trichosantha.[Pubmed:11170667]
J Nat Prod. 2001 Jan;64(1):60-4.
A new iridoid glycoside, deacetylalpinoside (2), was isolated from the aerial parts of Globularia trichosantha together with nine known iridoid glycosides: catalpol, 10-O-benzoyl-catalpol, aucubin, asperuloside, deacetylasperuloside, asperulosidic acid, scandoside, geniposidic acid, and alpinoside (1). From the underground parts of the same plant, two new bisiridoid glycosides, globulosides A (3) and B (4); a known iridoid glycoside, globularidin; a lignan glycoside, liriodendrin; and seven phenylethanoid glycosides, arenarioside, verbascoside (= acteoside), isoacteoside, crenatoside, Isocrenatoside, and trichosanthosides A and B, were isolated. Compounds 2-4 are new iridoids containing an 8,9 double bond representing a rare carbon skeleton. Their structures were established by spectroscopic methods.


