Iristectorin BCAS# 94396-09-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 94396-09-5 | SDF | Download SDF |
| PubChem ID | 138911431 | Appearance | Powder |
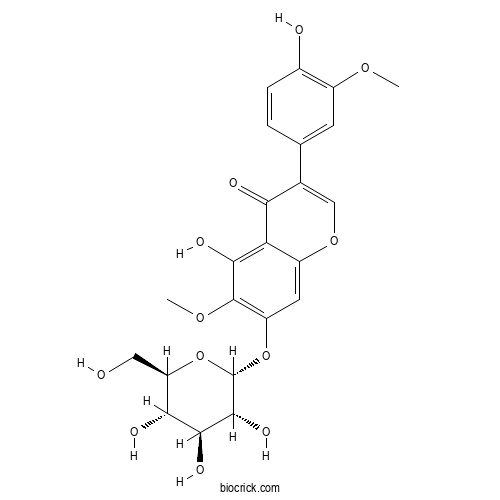
| Formula | C23H24O12 | M.Wt | 492.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-3-(4-hydroxy-3-methoxyphenyl)-6-methoxy-7-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | ||
| SMILES | COC1=C(C=CC(=C1)C2=COC3=CC(=C(C(=C3C2=O)O)OC)OC4C(C(C(C(O4)CO)O)O)O)O | ||
| Standard InChIKey | XVNKSNSGLVWSFS-IJLXDYNCSA-N | ||
| Standard InChI | InChI=1S/C23H24O12/c1-31-12-5-9(3-4-11(12)25)10-8-33-13-6-14(22(32-2)19(28)16(13)17(10)26)34-23-21(30)20(29)18(27)15(7-24)35-23/h3-6,8,15,18,20-21,23-25,27-30H,7H2,1-2H3/t15-,18-,20+,21-,23+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Iristectorin B Dilution Calculator

Iristectorin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0309 mL | 10.1543 mL | 20.3087 mL | 40.6174 mL | 50.7717 mL |
| 5 mM | 0.4062 mL | 2.0309 mL | 4.0617 mL | 8.1235 mL | 10.1543 mL |
| 10 mM | 0.2031 mL | 1.0154 mL | 2.0309 mL | 4.0617 mL | 5.0772 mL |
| 50 mM | 0.0406 mL | 0.2031 mL | 0.4062 mL | 0.8123 mL | 1.0154 mL |
| 100 mM | 0.0203 mL | 0.1015 mL | 0.2031 mL | 0.4062 mL | 0.5077 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Vavain
Catalog No.:BCX0504
CAS No.:199996-77-5
- 6-Demethoxyirigenin
Catalog No.:BCX0503
CAS No.:1348833-10-2
- Oxyphyllenodiol A
Catalog No.:BCX0502
CAS No.:363610-30-4
- Teuhetenone A
Catalog No.:BCX0501
CAS No.:152481-80-6
- Isocrenatoside
Catalog No.:BCX0500
CAS No.:221895-09-6
- Erythrinin E
Catalog No.:BCX0499
CAS No.:2731101-51-0
- Crenatoside
Catalog No.:BCX0498
CAS No.:61276-16-2
- Curdionolide B
Catalog No.:BCX0497
CAS No.:1190225-68-3
- 7,4'-Di-O-methylaromadendrin
Catalog No.:BCX0496
CAS No.:41515-76-8
- Sanggenol C
Catalog No.:BCX0495
CAS No.:174423-32-6
- Rugulolide D
Catalog No.:BCX0494
CAS No.:3002032-69-8
- Sanggenon E
Catalog No.:BCX0493
CAS No.:81381-69-3
- Methylconiferin
Catalog No.:BCX0506
CAS No.:883150-46-7
- 1β-Hydroxy-8α-methoxyeremophila-7(11),9-dien-12,8β-olide
Catalog No.:BCX0507
CAS No.:849700-45-4
- (S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-phenylheptan-3-one
Catalog No.:BCX0508
CAS No.:1220110-76-8
- Moracin N
Catalog No.:BCX0509
CAS No.:135248-05-4
- D-Mannosamine hydrochloride
Catalog No.:BCX0510
CAS No.:5505-63-5
- D-Galactosamine hydrochloride
Catalog No.:BCX0511
CAS No.:1772-03-8
- N-acetyl-D-galactosamine
Catalog No.:BCX0512
CAS No.:1811-31-0
- N-Acetyl-D-mannosamine
Catalog No.:BCX0513
CAS No.:7772-94-3
- Xanthomicrol
Catalog No.:BCX0514
CAS No.:16545-23-6
- 5,7-Dihydroxy-3,8,3',4'-tetramethoxyflavone
Catalog No.:BCX0515
CAS No.:42923-42-2
- 5,4'-Dihydroxy-6,7,8,3'-tetramethoxyflavone
Catalog No.:BCX0516
CAS No.:16520-78-8
- Tetrahydroauroglaucin
Catalog No.:BCX0517
CAS No.:40434-07-9
TMT and PRM Based Quantitative Proteomics to Explore the Protective Role and Mechanism of Iristectorin B in Stroke.[Pubmed:37894877]
Int J Mol Sci. 2023 Oct 15;24(20):15195.
Stroke is a serious disease caused by the rupture or blockage of the cerebrovascular system. Its pathogenesis is complex and involves multiple mechanisms. Iristectorin B is a natural isoflavone that has certain anti stroke effects. In this study, an in vitro stroke injury model of glyoxylate deprivation was established using PC12 cells, which was used to evaluate the anti-stroke activity of Iristectorin B in ejecta stem. The results showed that Iristectorin B, a natural isoflavone derived from Dried Shoot, significantly reduced the damage to PC12 cells caused by oxygen glucose deprivation/reoxygenation, decreased apoptosis, enhanced cell survival and reduced Ca(2+), LDH and ROS levels. The results showed that Iristectorin B had a significant protective effect on Na(2)S(2)O(4)-injured PC12 cells, and the mechanism may be related to the protective effect of neurons in the brain. After protein extraction and various analyses were performed, a series of cutting-edge technologies were organically combined to study the quantitative proteome of each group. Differential proteins were then analyzed. According to the protein screening principle, ferroptosis-related proteins were most closely associated with stroke. The differential proteins associated with ferroptosis screened were SLC3A2, TFR1 and HMOX1, with HMOX1 being the most significantly elevated and reduced via dosing. Iristectorin B may act as a protective agent against stroke by regulating ferroptosis, and SLC3A2, TFR1 and HMOX1 may serve as potential diagnostic biomarkers for stroke, providing additional evidence to support the importance of ferroptosis in stroke.
Identification and characterization of the chemical components of Iris tectorum Maxim. and evaluation of their nitric oxide inhibitory activity.[Pubmed:33001505]
Rapid Commun Mass Spectrom. 2021 Jan 15;35(1):e8959.
RATIONALE: Iris tectorum Maxim. is a traditional medicinal herb that is commonly used to treat inflammatory conditions. The present study investigated the fragmentation patterns of isoflavone glycosides and their qualitative analysis. In addition, lipopolysaccharide (LPS)-induced RAW264.7 macrophages were used to evaluate the anti-inflammatory properties of I. tectorum Maxim. samples collected at different time points during the year. METHODS: High-performance liquid chromatography/quadrupole time-of-flight tandem mass spectrometry (HPLC/QTOF-MS/MS) and HPLC with diode-array detection were employed for qualitative and quantitative analysis. The fragmentation patterns of the isoflavones were observed in negative electrospray ionization mode with collision-induced dissociation (CID). Their anti-inflammatory activity was assessed via nitric oxide (NO) production in LPS-treated RAW264.7 macrophages. RESULTS: A total of 15 chemical components were observed and tentatively identified using HPLC/QTOF-MS/MS. At low collision energy, the relative abundances of the aglycone radical anions Y(0) (-) , [Y(0) - H](-*) , [Y(0) - CH(3) ](-*) and [Y(0) - H- CH(2) ](-*) were used for the structural characterization of tectoridin and tectorigenin-4'-O-beta-D-glucoside. The radical ions [Y(0) - CH(3) ](-*) and [Y(0) - H - 2CH(3) ](-*) were also employed to differentiate between iristectorin A and Iristectorin B based upon their high-energy CID spectra. Levels of 9.02 mg/g of tectoridin and 1.04 mg/g of tectorigenin were found in samples collected in June, which exhibited 69.7% NO inhibitory activity. CONCLUSIONS: The characteristic fragmentation patterns enabled us to reliably identify isoflavone glycosides. The results of the quantitative determination and NO inhibitory activity offer insight into the optimal I. tectorum Maxim. harvesting time.
A novel strategy to evaluate the quality of herbal products based on the chemical profiling, efficacy evaluation and pharmacokinetics.[Pubmed:30196209]
J Pharm Biomed Anal. 2018 Nov 30;161:326-335.
The purpose of this study was to establish a chemical profiling method to compare the chemical composition of herbal products by using extracts of Belamcandae Rhizoma(EBR) extracted with different polarity solvent as an example, and evaluate the quality of EBR based on the analysis of chemical profiling, efficacy evaluation and pharmacokinetics. As seen from the results of chemical profiling, the PCA and PLS-DA score plot indicated that the dots of Belamcandae Rhizoma water extracts were separated from ethanol extracts obviously, which suggested significant differences of chemical profiling existing in the different solvent extracts. The PCA and PLS-DA loading plot illustrated that the main compounds contributing to chemical profiling differences were tectoridin(TD), Iristectorin B(IT B), iridin(ID), tectorigenin(TG), irigenin(IG), iristectorigein A(IG A), dichotomitin(DT) and irisflorentin(IF). Furthermore, the results of HPLC analysis demonstrated that the contents of these main compounds in ethanol extracts were significantly higher than that in water extracts (P < 0.01). Both the pharmacological and hematoxylin-eosin staining studies indicated that the ethanol extracts of Belamcandae Rhizoma had a better therapeutic effect than water extracts in oral ulcer model rats (P<0.01). It is suggested that the ethanol extracts were beneficial to the absorption and bioavailability of TG which was one of the most important bioactive compounds of Belamcandae Rhizoma in pharmacokinetic study in rats. This work provided a novel method to optimize the extraction process of EBR and related herbal products. Compared with the conventional chemical fingerprint methodology, the approach proposed above is not only a powerful tool to identify efficacy-related components for the quality evaluation, but also can be used to predict the therapeutic efficacy of herbal products.
Characterization and determination of the major constituents in Belamcandae Rhizoma by HPLC-DAD-ESI-MS(n).[Pubmed:21715119]
J Pharm Biomed Anal. 2011 Sep 10;56(2):304-14.
Belamcandae Rhizoma, derived from the rhizome of Belamcanda chinensis (L.) DC., has been used as traditional Chinese medicine for the treatment of coughing and pharyngitis. However, there have been few studies dealing with the systematic analysis of the bioactive constituents in Belamcandae Rhizoma. In this work, high performance liquid chromatography-diode array detection-electrospray ionization multiple-stage mass spectrometry (HPLC-DAD-ESI-MS(n)) combined with liquid chromatography-time of flight-mass spectrometry (HPLC-TOF/MS) was established for profiling and characterization of multi-constituent in Belamcandae Rhizoma. The ESI-MS(n) fragmentation behaviors of the authentic references were proposed for aiding the structural identification of components in the extract. Thirty-five flavonoids, including 30 isoflavones and five xanthones, were identified or tentatively identified by comparing their retention times, UV and MS spectra with those of authentic compounds or literature data. Twelve of the identified compounds (neomangiferin, mangiferin, tectoridin, Iristectorin B, iristectorin A, iridin, tectorigenin, iristectorigenin A, irigenin, irisflorentin, irilone and dichtomitin) were determined by HPLC-DAD using a C(18) column. The results indicated that the developed analysis method could be employed as a rapid, effective technique for structural characterization of chemical constituents in herbal medicine. This work is expected to provide comprehensive information for the quality evaluation of Belamcandae Rhizoma, which would be a valuable reference for the further study and development of this herb and related medicinal products.
PREPARATIVE ISOLATION AND PURIFICATION OF CHEMICAL CONSTITUENTS OF BELAMCANDA BY MPLC, HSCCC AND PREP-HPLC.[Pubmed:21552369]
J Liq Chromatogr Relat Technol. 2011;34(4):241-257.
Combined with medium-pressure liquid chromatography (MPLC) and preparative high-pressure liquid chromatography (Prep-HPLC), high-speed countercurrent chromatography (HSCCC) was successfully applied for separation and purification of isoflavonoids from the extract of belamcanda. HSCCC separation was performed on a two-phase solvent system composed of methyl tert-butyl ether -ethyl acetate - n-butyl alcohol - acetonitrile -0.1% aqueous trifluoroacetic acid at a volume radio of 1:2:1:1:5. Semi-purified peak fractions from HSCCC separation were further purified by Prep-HPLC. Nine well-separated fractions were analyzed by HPLC-UV absorption spectrometry to determine their purities and characterized with ESI-MS(n). Except for peaksland VII (unknown) seven compounds were identified as apocynin (peak II), mangiferin (peak III), 7-O-methylmangiferin (peak IV), hispidulin (peak V), 3'-hydroxyltectoridin (peak VI), Iristectorin B (peak VII), isoiridin (peak IX).
Ionic liquid based ultrasonic assisted extraction of isoflavones from Iris tectorum Maxim and subsequently separation and purification by high-speed counter-current chromatography.[Pubmed:21444254]
J Chromatogr B Analyt Technol Biomed Life Sci. 2011 Apr 15;879(13-14):975-80.
We developed an ionic liquid based ultrasonic assisted extraction (ILUAE) method for the extraction of the three isoflavones, namely tectoridin, Iristectorin B and iristectorin A from Iris tectorum Maxim of the Iridaceae family. Three kinds of 1-alkyl-3-methylimidazolium ionic liquids with different alkyl chain and anion were investigated. The results indicated that ionic liquids (ILs) showed remarkable effects on the extraction yield of isoflavones. In addition, the ILUAE, including several ultrasonic parameters, such as the concentration, extraction time and solvent to solid ratio have been optimized. Under these optimal conditions (e.g., with 30 min extraction time and the solvent to solid ratio of 30 ml/g), this approach gained the highest extraction yields of tectoridin (37.45 mg/g), Iristectorin B (2.88 mg/g) and iristectorin A (5.28 mg/g). Meanwhile, tectoridin, Iristectorin B and iristectorin A in the ILUAE extract were separated and purified successfully through the high-speed counter-current chromatography (HSCCC) with a two-phase solvent system consisting of n-butanol-water (1:1, v/v). The additional advantage of this approach is that 60.21 mg tectoridin, 4.33 mg Iristectorin B and 8.24 mg iristectorin A with more than 95.0% purities have been obtained from 400 mg ILUAE extract of I. tectorum within 5 h and one-step elution under the most optimized conditions (e.g., a flow rate of 2.0 ml/min, 900 rpm and the wavelengh of 280 nm). The obtained fractions were successfully analyzed by HPLC and identified by (1)H-NMR and (13)C-NMR.
Ultrasound-assisted extraction of five isoflavones from Iris tectorum Maxim.[Pubmed:32288612]
Sep Purif Technol. 2011 Mar 24;78(1):49-54.
This study investigated the use of ultrasound-assisted extraction (UAE) to improve the extraction efficiency of the classical solvent extraction techniques such as maceration extraction (ME) and soxhlet extraction (SE) to extract five isoflavones (tectoridin, Iristectorin B, iristectorin A, tectorigenin and iristectorigenin A) from Iris tectorum. The effects of various factors such as extraction solvent, solvent concentration, temperature, solvent to solid ratio, ultrasound power, extraction time and particle size on the yield of target components were investigated. The optimal UAE conditions found were: 70% (v/v) methanol solution, temperature 45 degrees C, solvent to solid ratio 15 ml/g, ultrasound power 150 W, extraction time 45 min and particle size 60-80 mesh. The results indicated that compared with ME at 18 h and SE at 6 h, UAE gave the highest extraction yields of tectoridin, Iristectorin B, iristectorin A, tectorigenin, iristectorigenin A and total isoflavones at 45 min. The results indicated that UAE was an alternative method for extracting isoflavones from I. tectorum.
[Chemical constituents of Iris dichotoma].[Pubmed:21355241]
Zhongguo Zhong Yao Za Zhi. 2010 Dec;35(23):3168-71.
OBJECTIVE: To study the chemical constituents in the rhizoma of Iris dichotoma. METHOD: The chemical constituents were isolated by various column chromatographic methods. The structures of the compounds were elucidated on the basis of physiochemical properties and spectral analysis. RESULT: Eleven compounds, hispidulin (1), rhamnocitrin (2), iristectorigenin A (3), 4', 5, 7, 8-tetrahydroxy-6-methoxy isoflavone (4), 6-hydroxybiochanin A (5), Iristectorin B (6), iristectorigenin A (7), kaempferol-7-methyl ether (8), tamarixetin-7-glucoside (9), iristectorin A (10), 3', 3, 5-trihydroxy-4', 7-dimethoxy-flavone-3-O-beta-D-galactopyranoside (11) were isolated and identified. CONCLUSION: Compounds 1-11 were obtained from this plant for the first time.
[Studies on the isoflavonoids of Iris tectorum].[Pubmed:20034214]
Zhong Yao Cai. 2009 Sep;32(9):1392-4.
OBJECTIVE: To study the isoflavonoids of Iris tectorum. METHODS: The compounds were isolated by columm chromatography on silica gel and purified by Sephadex LH-20. Structures of these compounds were elucidated by spectral analysis and phytochemical properties. RESULTS: Six isoflavonoids were isolated from Iris tectorum, which were tectoridin (I), tectorigenin (II), iristectorin A (III), iristectorigenin A (IV), Iristectorin B (V), 5,7,4'-trihydroxy-6,3'-dimethoxy isoflvone (VI). CONCLUSION: Compound VI is isolated from this plant for the first time.
Phenolic constituents of the rhizomes of the Thai medicinal plant Belamcanda chinensis with proliferative activity for two breast cancer cell lines.[Pubmed:15787436]
J Nat Prod. 2005 Mar;68(3):361-4.
From the rhizomes of Belamcanda chinensis, three new compounds, belalloside A (1), belalloside B (2), and belamphenone (3), along with 13 known compounds, resveratrol (4), iriflophenone (5), irisflorentin (6), tectorigenin (7), irilin D (8), tectoridin (9), iristectorin A (10), Iristectorin B (11), hispiduloside, androsin, irigenin, iridin, and jaceoside, have been isolated and characterized. Isolates were evaluated for their cell proliferation stimulatory activity against the MCF-7 and T-47D human breast cancer cell lines. Along with 4, 5, 7, and 9, 3 was shown to stimulate not only MCF-7 but also T-47D human breast cancer cell proliferation.


