(+)-MelleinCAS# 62623-84-1 |
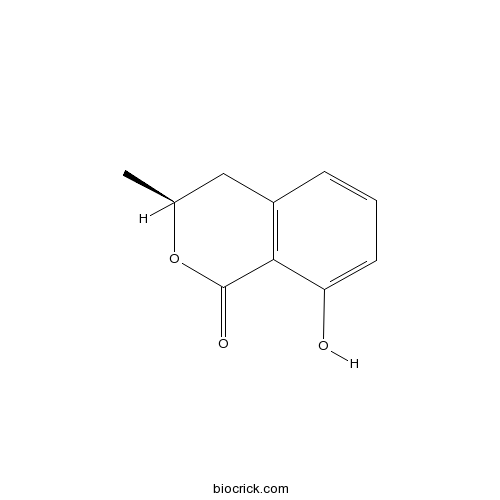
- Mellein
Catalog No.:BCN4785
CAS No.:480-33-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 62623-84-1 | SDF | Download SDF |
| PubChem ID | 10921069 | Appearance | Powder |
| Formula | C10H10O3 | M.Wt | 178.18 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S)-8-hydroxy-3-methyl-3,4-dihydroisochromen-1-one | ||
| SMILES | CC1CC2=C(C(=CC=C2)O)C(=O)O1 | ||
| Standard InChIKey | KWILGNNWGSNMPA-LURJTMIESA-N | ||
| Standard InChI | InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-6/h2-4,6,11H,5H2,1H3/t6-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (+)-Mellein is a natural product from Stevia lucida Lagasca. |
| Structure Identification | Avances En Química, 2013,8(3):145-151.Isolation and characterization of (+)-mellein, the first isocoumarin reported in Stevia genus[Reference: WebLink]
|

(+)-Mellein Dilution Calculator

(+)-Mellein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6123 mL | 28.0615 mL | 56.123 mL | 112.246 mL | 140.3076 mL |
| 5 mM | 1.1225 mL | 5.6123 mL | 11.2246 mL | 22.4492 mL | 28.0615 mL |
| 10 mM | 0.5612 mL | 2.8062 mL | 5.6123 mL | 11.2246 mL | 14.0308 mL |
| 50 mM | 0.1122 mL | 0.5612 mL | 1.1225 mL | 2.2449 mL | 2.8062 mL |
| 100 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1225 mL | 1.4031 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4,6,7-Trimethoxy-5-methylcoumarin
Catalog No.:BCN4166
CAS No.:62615-63-8
- 2',4'-Dihydroxy-3'-methoxyacetophenone
Catalog No.:BCN7535
CAS No.:62615-26-3
- Oxiracetam
Catalog No.:BCC5447
CAS No.:62613-82-5
- H-HoCys-OH
Catalog No.:BCC3231
CAS No.:626-72-2
- 2,4-Dihydroxypyridine
Catalog No.:BCC8500
CAS No.:626-03-9
- Neocyclomorusin
Catalog No.:BCN3601
CAS No.:62596-35-4
- Cyclomorusin
Catalog No.:BCN4610
CAS No.:62596-34-3
- Morusin
Catalog No.:BCN4165
CAS No.:62596-29-6
- 11R,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1392
CAS No.:62574-30-5
- Captopril
Catalog No.:BCC2140
CAS No.:62571-86-2
- H-D-Phe(4-Br)-OH
Catalog No.:BCC3158
CAS No.:62561-74-4
- Isodihydrofutoquinol A
Catalog No.:BCN6691
CAS No.:62560-95-6
- 11S,12-Dihydroxyspirovetiv-1(10)-en-2-one
Catalog No.:BCN1391
CAS No.:62623-86-3
- Ro 106-9920
Catalog No.:BCC7175
CAS No.:62645-28-7
- SIB 1893
Catalog No.:BCC6970
CAS No.:6266-99-5
- 1-Hydroxy-2-methylanthraquinone
Catalog No.:BCN3478
CAS No.:6268-09-3
- Handelin
Catalog No.:BCN2953
CAS No.:62687-22-3
- Saikosaponin F
Catalog No.:BCN2776
CAS No.:62687-63-2
- H-D-Arg-OH.HCl
Catalog No.:BCC2869
CAS No.:627-75-8
- Dioctanoylglycol
Catalog No.:BCC6662
CAS No.:627-86-1
- NF 449
Catalog No.:BCC7043
CAS No.:627034-85-9
- Dihydrolycorine
Catalog No.:BCN2475
CAS No.:6271-21-2
- SKF38393 HCl
Catalog No.:BCC6526
CAS No.:62717-42-4
- 4-Amino-N-methylbenzamide
Catalog No.:BCC8685
CAS No.:6274-22-2
Isolation of anticancer and anti-trypanosome secondary metabolites from the endophytic fungus Aspergillus flocculus via bioactivity guided isolation and MS based metabolomics.[Pubmed:30658264]
J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Feb 1;1106-1107:71-83.
This study aims to identify bioactive anticancer and anti-trypanosome secondary metabolites from the fermentation culture of Aspergillus flocculus endophyte assisted by modern metabolomics technologies. The endophyte was isolated from the stem of the medicinal plant Markhamia platycalyx and identified using phylogenetics. Principle component analysis was employed to screen for the optimum growth endophyte culturing conditions and revealing that the 30-days rice culture (RC-30d) provided the highest levels of the bioactive agents. To pinpoint for active chemicals in endophyte crude extracts and successive fractions, a new application of molecular interaction network is implemented to correlate the chemical and biological profiles of the anti-trypanosome active fractions to highlight the metabolites mediating for bioactivity prior to purification trials. Multivariate data analysis (MVDA), with the aid of dereplication studies, efficiently annotated the putatively active anticancer molecules. The small-scale RC-30d fungal culture was purified using high-throughput chromatographic techniques to yield compound 1, a novel polyketide molecule though inactive. Whereas, active fractions revealed from the bioactivity guided fractionation of medium scale RC-30d culture were further purified to yield 7 metabolites, 5 of which namely cis-4-hydroxymellein, 5-hydroxymellein, diorcinol, botryoisocoumarin A and (+)-Mellein, inhibited the growth of chronic myelogenous leukemia cell line K562 at 30muM. 3-Hydroxymellein and diorcinol exhibited a respective inhibition of 56% and 97% to the sleeping sickness causing parasite Trypanosoma brucei brucei. More interestingly, the anti-trypanosomal activity of A. flocculus extract appeared to be mediated by the synergistic effect of the active steroidal compounds i.e. ergosterol peroxide, ergosterol and campesterol. The isolated structures were elucidated by using 1D, 2D NMR and HR-ESIMS.
Pestalotiopisorin B, a new isocoumarin derivative from the mangrove endophytic fungus Pestalotiopsis sp. HHL101.[Pubmed:30623682]
Nat Prod Res. 2019 Jan 9:1-6.
A new isocoumarin derivative pestalotiopisorin B (1), along with six known compounds, (R)-(-)- (+)-Mellein methyl ether (2), pestalotiopyrone G (3), (R)-mevalonolactone (4), pestalotiollides A-B (5-6) and pestalotiopsol A(7) were isolated from Pestalotiopsis sp., an endophytic fungus obtained from Chinese mangrove plant Rhizophora stylosa. Their structures were elucidated unambiguously by the comprehensive analysis of extensive spectroscopic data. Compound 1 exhibited modest antibacterial activity against Escherichia coli and Pseudomonas aeruginosa with 12.5 mug/ml, 50 mug/ml, respectively. Compound 4 showed moderate calcineurin inhibitory activity towards p-nitrophenyl phosphate (IC50 =134.29 +/- 5.377 muM).
Secondary metabolites produced by Sardiniella urbana, a new emerging pathogen on European hackberry.[Pubmed:29848074]
Nat Prod Res. 2018 May 30:1-8.
In this study the production of secondary metabolites by a virulent strain of Sardiniella urbana, a recently described pathogen originally found on declining European hackberry trees in Italy, was investigated for the first time. Chemical analysis of the culture filtrate extracts led to the isolation of three well known compounds as R-(-)-mellein and (3R,4R)-and (3R,4S)-4-hydroxy (+)-Melleins which were identified by spectroscopic methods (essentially NMR and ESIMS). The isolated compounds were tested for their phytotoxic, antifungal and zootoxic activities. Among them, only R-(-)-mellein was found to be active.
5-Methylmellein is a novel inhibitor of fungal sirtuin and modulates fungal secondary metabolite production.[Pubmed:29794367]
J Gen Appl Microbiol. 2018 Nov 9;64(5):240-247.
Sirtuin is an NAD(+)-dependent histone deacetylase that is highly conserved among prokaryotes and eukaryotes. Sirtuin deacetylates histones and non-histone proteins, and it is involved in fungal growth and secondary metabolite production. Here, we screened 579 fungal culture extracts that inhibited the histone deacetylase activity of Sirtuin A (SirA), produced by the fungus Aspergillus nidulans. Eight fungal strains containing three Ascomycota, two Basidiomycota and three Deuteromycetes produced SirA inhibitors. We purified the SirA inhibitor from the culture broth of Didymobotryum rigidum JCM 8837, and identified it as 5-methylmellein-a known polyketide. This polyketide and its structurally-related compound, (+)-Mellein, inhibited SirA activity with IC50 of 120 and 160 muM, respectively. Adding 5-methylmellein to A. nidulans cultures increased secondary metabolite production in the medium. The metabolite profiles were different from those obtained by adding other sirtuin inhibitors nicotinamide and sirtinol to the culture. These results indicated that 5-methylmellein modulates fungal secondary metabolism, and is a potential tool for screening novel compounds derived from fungi.
Natural Products of Picea Endophytes from the Acadian Forest.[Pubmed:28398744]
J Nat Prod. 2017 May 26;80(5):1475-1483.
Endophytes of healthy needles were collected from Picea rubens (red spruce) and P. mariana (black spruce) in a survey of southeastern New Brunswick, Canada. Four endophyte strains were selected for further investigation based on the production of biologically active extracts from culture filtrates during screening as well as phylogenetic relationship to species known to produce natural products or taxonomic novelty. A novel endophyte within the family Rhytismataceae produced two new dihydropyrones (1 and 2) as major metabolites together with phthalides (3 and 4), isocoumarins (5 and 6), and tyrosol (7). Lachnum cf. pygmaeum synthesized a new chlorinated para-quinone, chloromycorrhizinone A (8), and the nematicidal compounds (1'Z)-dechloromycorrhizin A (9), mycorrhizin A (10), and chloromycorrhizin A (11). A new isocoumarin (12) and four related structures (13-16) were isolated from an undescribed taxon in the Mycosphaerellaceae. The known antifungal metabolites cryptosporiopsin (17), 5-hydroxycryptosporiopsin (18), (+)-cryptosporiopsinol (19), and (+)-Mellein (20) were produced by Pezicula sporulosa. Phylogenetically diverse conifer endophytes from the Acadian forest continue to be a productive source of new biologically active natural products.
Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste.[Pubmed:28372238]
Food Chem. 2017 Aug 15;229:734-742.
Vinegar is an inexpensive commodity, and economic considerations require that a relatively low-cost raw material be used for its production. An investigation into the use of a new, alternative substrate - pineapple waste - is described. This approach enables the utilization of the pineapple's (Ananas comosus) peels and core, which are usually discarded during the processing or consumption of the fruit. Using physical and enzymatic treatments, the waste was saccharified, and the resulting substrate was fermented with Saccharomyces cerevisiae for 7-10days under aerobic conditions at 25 degrees C. This resulted in an alcohol yield of approximately 7%. The alcoholic medium was then used as a seed broth for acetic fermentation using Acetobacter aceti as the inoculum for approximately 30days at 32 degrees C to obtain 5% acetic acid. Samples were analyzed at the beginning and end of the acetification cycle to assess the volatile and fixed compounds by GC-MS and UHPLC-QTOF-MS. The metabolomic analysis indicated that l-lysine, (+)-Mellein, and gallic acid were significantly more concentrated in the pineapple vinegar than in the original wine. Higher alcohols, aldehydes, and ketones characterized the aroma of the final pineapple vinegar, whilst off-flavors were significantly reduced relative to the initial wine. This study is the first to highlight the metabolite profile of fruit vinegar with a slight floral aroma profile derived from pineapple waste. The potential to efficiently reduce the post-harvest losses of pineapple fruits by re-using them for products with added food values is also demonstrated.
Secondary metabolites of endophytic Xylaria species with potential applications in medicine and agriculture.[Pubmed:27896581]
World J Microbiol Biotechnol. 2017 Jan;33(1):15.
Fungal endophytes are important sources of bioactive secondary metabolites. The genus Xylaria Hill (ex Schrank, 1789, Xylariaceae) comprises various endophytic species associated to both vascular and non vascular plants. The secondary metabolites produced by Xylaria species include a variety of volatile and non-volatile compounds. Examples of the former are sesquiterpenoids, esters, and alcohols, among others; and of the latter we find terpenoids, cytochalasins, (+)-Mellein, alkaloids, polyketides, and aromatic compounds. Some of these metabolites have shown potential activity as herbicides, fungicides, and insecticides; others possess antibacterial, antimalarial, and antifungal activities, or alpha-glucosidase inhibitory activity. Thus metabolites from Xylaria are promising compounds for applications in agriculture for plague control as biopesticides, and biocontrol agents; and in medicine, for example as drugs for the treatment of infectious and non-infectious diseases. This review seeks to show the great value of the secondary metabolites of Xylaria, particularly in the agriculture and medicine fields.
High-performance liquid chromatography-tandem mass spectrometry method for simultaneous detection of ochratoxin A and relative metabolites in Aspergillus species and dried vine fruits.[Pubmed:27442910]
Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2016 Aug;33(8):1355-66.
A simple, sensitive and reliable quantification and identification method was developed for simultaneous analysis of ochratoxin A (OTA) and its related metabolites ochratoxin alpha (OTalpha), ochratoxin B (OTB) and (+)-Mellein. The method was assessed by spiking analytes into blank culture media and dried vine fruits. Performance was tested in terms of accuracy, selectivity and repeatability. The method involves an ultrasonic extraction step for culture samples using methanol aqueous solution (7:3, v/v); the mycotoxin is quantified by high-performance liquid chromatography coupled with electrospray ionisation and triple quadrupole mass spectrometry (LC-ESI-MS/MS). The recoveries were 74.5-108.8%, with relative standard deviations (RSDs) of 0.4-8.4% for fungal culture. The limits of detection (LODs) were in the range of 0.03-0.87 mug l(-)(1), and the limits of quantification (LOQs) ranged from 0.07 to 2.90 mug l(-)(1). In addition, the extraction solutions and clean-up columns were optimised specifically for dried vine fruit samples to improve the performance of the method. Methanol-1% sodium bicarbonate extraction solution (6:4, v/v) was determined to be the most efficient. Solid-phase extraction (SPE) was performed as a clean-up step prior to HPLC-MS/MS analysis to reduce matrix effects. Recoveries ranged from 80.1% to 110.8%. RSDs ranged from 0.1% to 3.6%. LODs and LOQs ranged from 0.06 to 0.40 mug kg(-)(1) and from 0.19 to 1.20 mug kg(-)(1), respectively. The analytical method was established and used to identify and quantify OTA and related compounds from Aspergillus carbonarius and Aspergillus ochraceus in cultures and dried vine fruits. The results showed that A. carbonarius produced OTalpha, OTB and OTA, whereas A. ochraceus produced OTB, OTA and (+)-Mellein after 7 days of cultivation. Of 30 commercial samples analysed, 10 were contaminated with ochratoxins; OTB, OTalpha and (+)-Mellein were also detected in different samples.


