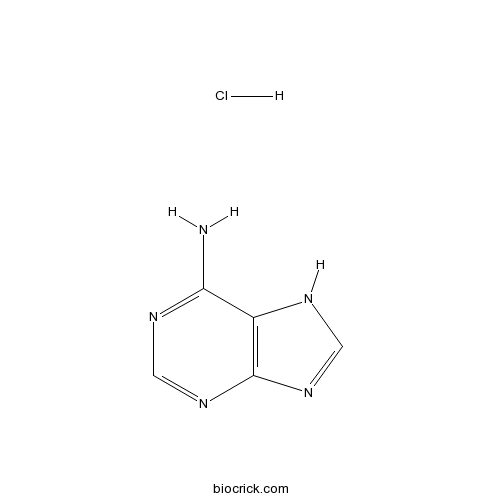
Adenine HClAdenine receptor agonist CAS# 2922-28-3 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- DPH
Catalog No.:BCC1538
CAS No.:484049-04-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2922-28-3 | SDF | Download SDF |
| PubChem ID | 76219 | Appearance | Powder |
| Formula | C5H6ClN5 | M.Wt | 171.59 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 8 mg/mL (46.62 mM) in DMSO | ||
| Chemical Name | 7H-purin-6-amine;hydrochloride | ||
| SMILES | [H+].[Cl-].Nc1ncnc2nc[nH]c12 | ||
| Standard InChIKey | UQVDQSWZQXDUJB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H5N5.ClH/c6-4-3-5(9-1-7-3)10-2-8-4;/h1-2H,(H3,6,7,8,9,10);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Adenine HCl Dilution Calculator

Adenine HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.8278 mL | 29.1392 mL | 58.2785 mL | 116.5569 mL | 145.6961 mL |
| 5 mM | 1.1656 mL | 5.8278 mL | 11.6557 mL | 23.3114 mL | 29.1392 mL |
| 10 mM | 0.5828 mL | 2.9139 mL | 5.8278 mL | 11.6557 mL | 14.5696 mL |
| 50 mM | 0.1166 mL | 0.5828 mL | 1.1656 mL | 2.3311 mL | 2.9139 mL |
| 100 mM | 0.0583 mL | 0.2914 mL | 0.5828 mL | 1.1656 mL | 1.457 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Not available.
Adenine HCl, a purine derivative and a nucleobase, plays crucial roles in substantial biochemistry processes in vivo, including cellular respiration, formation of the energy-rich adenosine triphosphate (ATP), the cofactors nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD) as well as protein synthesis. In addition, Adenine HCl also serves as a chemical component of DNA and RNA. Adenosine triphosphate is used in cellular metabolism as one of the basic methods of transferring chemical energy between chemical reactions. [1]
In vitro: The cyto-protective effect of Adenosine was measured using an in vitro model of acute tubular necrosis in rat kidney tubular cells. The finding suggested that Adenosine at the concentration of 100 M could significantly decrease cellular injury. The EC50 value of Adenosine was detected to be 14 M. [2]
In vivo: A study was performed to investigate the effects of dietary adenine on fatty liver induced by orotic acid (OA) in rats. 1% OA-supplemented diets with/ without 0.25% adenine was administered to rats for 10 days. Enzyme assay kits were then applied to measure serum lipid profiles of tested rats, such as liver lipid concentrations in different treatment groups. Moreover, the activities of fatty acid synthase (FAS) and fatty acid β-oxidation were also detected. The findings suggested that addition of adenine to the diet offset the effect of OA and reversed promotion of liver TG content to basal level. Administration of Adenine also inhibited FAS activities in rat liver. In conclusion, the ameliorating of fatty liver in adenine-treated rats was associated with the reduction of FAS activities accompanied with the increase of mitochondrial fatty acid β-oxidation and the promotion of serum lipid secretion from the hepatic tissue into the bloodstream. [1]
Clinical trials: So far, no clinical trial has been conducted.
References:
[1]Buang Y. Dietary adenine alleviates fatty liver induced by orotic acid. Indo. J. Chem. 2010; 10 (3): 363 - 369.
[2] Módis K, Ger D, Nagy N, Szoleczky P, Tóth ZD and Szabó C. Cytoprotective effects of adenosine and inosine in an in vitro model of acute tubular necrosis. Br J Pharmacol. 2009 Nov; 158(6): 1565–8.
- Methylprednisolone hemisuccinate
Catalog No.:BCC9044
CAS No.:2921-57-5
- 3',6'-Bis(diethylamino)-2-(4-nitrophenyl)spiro[isoindole-1,9'-xanthene]-3-one
Catalog No.:BCC8597
CAS No.:29199-09-5
- Shegansu B
Catalog No.:BCN3381
CAS No.:291535-65-4
- [Ala11,22,28]VIP
Catalog No.:BCC5754
CAS No.:291524-04-4
- 1-Methoxyberberine
Catalog No.:BCN7373
CAS No.:29133-52-6
- (+)-Affinisine
Catalog No.:BCN3520
CAS No.:2912-11-0
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- Guanfacine
Catalog No.:BCC5180
CAS No.:29110-47-2
- Procyanidin B2
Catalog No.:BCN6315
CAS No.:29106-49-8
- Pinoresinol dimethyl ether
Catalog No.:BCN6767
CAS No.:29106-36-3
- Glipizide
Catalog No.:BCC3785
CAS No.:29094-61-9
- Cimiracemoside D
Catalog No.:BCN2789
CAS No.:290821-39-5
- H-DL-Phe(4-NO2)-OH
Catalog No.:BCC3279
CAS No.:2922-40-9
- L-Kynurenine
Catalog No.:BCC3899
CAS No.:2922-83-0
- Dehydrotrametenolic acid
Catalog No.:BCN2718
CAS No.:29220-16-4
- SB-3CT
Catalog No.:BCC5486
CAS No.:292605-14-2
- 25,26-Dihydroxyvitamin D3
Catalog No.:BCC4201
CAS No.:29261-12-9
- L-685,458
Catalog No.:BCC2344
CAS No.:292632-98-5
- Deoxyelephantopin
Catalog No.:BCN4655
CAS No.:29307-03-7
- Genipin-1-O-gentiobioside
Catalog No.:BCN5349
CAS No.:29307-60-6
- Licarbazepine
Catalog No.:BCC7794
CAS No.:29331-92-8
- Tetrahydropalmatine
Catalog No.:BCN6310
CAS No.:2934-97-6
- Ciclopirox
Catalog No.:BCC4899
CAS No.:29342-05-0
- Olean-12-ene-3,11-dione
Catalog No.:BCN5195
CAS No.:2935-32-2
In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dineculeotide from halloysite; a cylindrical mineral.[Pubmed:11695636]
J Microencapsul. 2001 Nov-Dec;18(6):713-22.
The use of halloysite clay as a low cost alternative to more traditional microencapsulation systems is reported. Halloysite is an alumino-silicate clay which demonstrates a predominately cylindrical geometry, uniquely characterized by a hollow core or series of voids with diameters ranging from 16-50 nm. These nanoscale-to-mesoscale microcylinders are capable of entrapping active agents within the core lumen as well as within any void spaces contained in the multilayered walls of the cylinder. Some of the active agents associated with the clay are also bound to the external surfaces of the clay. Delivery of the active agent is first by desorption of the active agent from the exterior surfaces and exposed ends of the microcylinders, and is followed by a second more prolonged phase dominated by pore diffusion from the ends of the cylinders. Halloysite is capable of retaining and releasing a range of active ingredients. Both hydrophilic and hydrophobic agents may be entrapped following appropriate pre-treatment of the clay to render it lipophilic. Here, a unique low cost alternative microcylindrical delivery system: the clay mineral halloysite, is investigated.
Ionization of adenine derivatives: EPR and ENDOR studies of X-irradiated adenine.HCl.1/2H2O and adenosine.HCl.[Pubmed:1332108]
Radiat Res. 1992 Sep;131(3):272-84.
Following X irradiation of adenine.HCl.H2O at 10 K, evidence for five distinct radical products was present in the EPR/ENDOR. (In both adenine.HCl.1/2H2O and adenosine.HCl, the adenine base is present in a cationic form as it is protonated at N1). From ENDOR data, radical R1, stable at temperatures up to 250 K, was identified as the product of net hydrogen loss from N1. This product, evidently formed by electron loss followed by proton loss, is equivalent to the radical cation of the neutral adenine base. Radical R2, unstable at temperatures above 60 K, was identified as the product of net hydrogen addition to N3, and evidently formed by electron addition followed by proton addition. Radicals R3-R5 could not be identified with certainty. Similar treatment of adenosine.HCl provided evidence for six identifiable radical products. Radical R6, stable to ca. 150 K, was identified as the result of net hydrogen loss from the amino group, and evidently was the product of electron loss followed by proton loss. Radical R7 was tentatively identified as the product of net hydrogen addition to C4 of the adenine base. Radical R8 was found to be the product of net hydrogen addition to C2 of the adenine base, and R9 was the product of net hydrogen addition to C8. Radical R10 was identified as the product of net hydrogen abstraction from C1' of the ribose, and R11 was an alkoxy radical formed from the ribose. With the exception of R11, all products were also found following irradiation at 65 K. Only radical R8 and R9 were stable at room temperature. Most notable is the different deprotonation behavior of the primary electron-loss products (radical R1 vs. R6) and the different protonation behavior of the primary electron-gain products (radical R2 vs. no similar product in adenosine.HCl). The major structural difference in the two crystals is the electrostatic environment of the adenine base. Therefore, this study provides further evidence that environmental influences are important in determining proton transfer processes.
Poly (9-(2-diallylaminoethyl)adenine HCl-co-sulfur dioxide) deposited on silica nanoparticles constructs hierarchically ordered nanocapsules: curcumin conjugated nanocapsules as a novel strategy to amplify guanine selectivity among nucleobases.[Pubmed:25569875]
Biosens Bioelectron. 2015 Jun 15;68:181-188.
Poly (9-(2-diallylaminoethyl)Adenine HCl-co-sulfur dioxide) (Poly A) deposited on silica nanoparticles self-assembles to form hierarchically ordered nanocapsules. These nanocapsules can be conjugated with curcumin. The curcumin-conjugated nanocapsules are found to be spherical in size and their size ranges between 200 and 600 nm. We found that curcumin conjugated with silica nanoparticles marginally shows a selectivity ( approximately 20%) for guanine over adenine, cytosine, thymine and uracil, but this selectivity is extraordinarily amplified to more than 500% in curcumin-conjugated nanocapsules prepared from the above procedure. FT-IR spectra along with lifetime measurements suggest that specific interaction between adenine moieties of Poly A nanocapsules and thymine/uracil does not affect the fluorescence of poly A nanocapsules. Thus, the sensitivity and selectivity for guanine estimation is due to hydrophobic interactions, which are assisted by the low water solubility of guanine as compared to the other nucleobases. The present method illustrates a wider linear dynamic range in the higher concentration range as compared to the reported methods. Finally, the degradation study proves that stability of curcumin is improved dramatically in such nanocapsules demonstrating that nanotechnology could be a viable method to improve selectivity of specific analyte and robustness of probe molecule during fluorescence based bio-sensing.


