KU-0060648Dual DNA-PK/PI3-K inhibitor, ATP-competitive CAS# 881375-00-4 |
- ETP-46464
Catalog No.:BCC3913
CAS No.:1345675-02-6
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- Daun02
Catalog No.:BCC1518
CAS No.:290304-24-4
- NU7441 (KU-57788)
Catalog No.:BCC3679
CAS No.:503468-95-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 881375-00-4 | SDF | Download SDF |
| PubChem ID | 11964036 | Appearance | Powder |
| Formula | C33H34N4O4S | M.Wt | 582.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in 2eq.HCl | ||
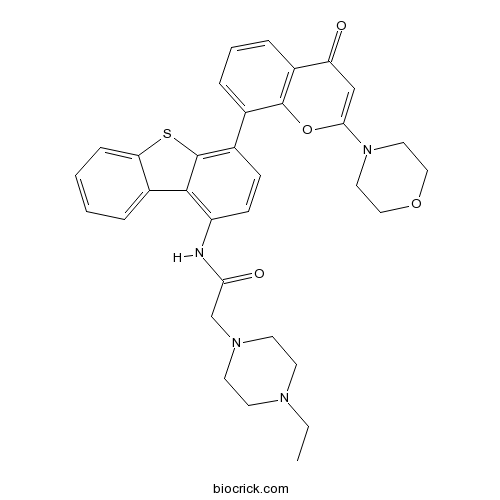
| Chemical Name | 2-(4-ethylpiperazin-1-yl)-N-[4-(2-morpholin-4-yl-4-oxochromen-8-yl)dibenzothiophen-1-yl]acetamide | ||
| SMILES | CCN1CCN(CC1)CC(=O)NC2=C3C4=CC=CC=C4SC3=C(C=C2)C5=CC=CC6=C5OC(=CC6=O)N7CCOCC7 | ||
| Standard InChIKey | AATCBLYHOUOCTO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C33H34N4O4S/c1-2-35-12-14-36(15-13-35)21-29(39)34-26-11-10-23(33-31(26)25-6-3-4-9-28(25)42-33)22-7-5-8-24-27(38)20-30(41-32(22)24)37-16-18-40-19-17-37/h3-11,20H,2,12-19,21H2,1H3,(H,34,39) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dual PI 3-K and DNA-PK inhibitor (IC50 values are <0.1, 0.5, 4 and 19 nM for PI 3-Kδ, PI 3-Kβ, PI 3-Kα and DNA-PK respectively). Inhibits proliferation of MCF7 cells in vitro and delays growth of MCF7 xenografts in mice. Also enhances CRISPR-Cas9-mediated homology-directed repair (HDR) efficiency ~2 to 4-fold, and decreases nonhomologous end-joining (NHEJ) frequency ~40%; maximum effect seen at 250 nM. |

KU-0060648 Dilution Calculator

KU-0060648 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7161 mL | 8.5806 mL | 17.1612 mL | 34.3224 mL | 42.903 mL |
| 5 mM | 0.3432 mL | 1.7161 mL | 3.4322 mL | 6.8645 mL | 8.5806 mL |
| 10 mM | 0.1716 mL | 0.8581 mL | 1.7161 mL | 3.4322 mL | 4.2903 mL |
| 50 mM | 0.0343 mL | 0.1716 mL | 0.3432 mL | 0.6864 mL | 0.8581 mL |
| 100 mM | 0.0172 mL | 0.0858 mL | 0.1716 mL | 0.3432 mL | 0.429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: KU-0060648 inhibited cellular DNA-PK autophosphorylation with IC50 values of 0.17 μmol/L (SW620 cells) and 0.019 μmol/L (MCF7 cells), and PI-3K–mediated AKT phosphorylation with IC50 values of 0.039 μmol/L (MCF7 cells) and more than 10 μmol/L (SW620 cells).
DNA double-strand breaks (DSB) are the most cytotoxic lesions induced by topoisomerase II poisons. Nonhomologous end joining (NHEJ) is a major pathway for DSB repair and requires DNA-dependent protein kinase (DNA-PK) activity. DNA-PK catalytic subunit (DNA-PKcs), which promotes cell survival and proliferation and is upregulated in many cancers, is structurally similar to PI-3K,. KU-0060648 is a dual inhibitor of DNA-PKand PI-3K in vitro.
In vitro: KU-0060648 was investigated in a panel ofhumanbreast and colon cancer cells. Five-day exposure to 1 μM KU-0060648 was found to inhibite cell proliferation by more than 95% in MCF7 cells but only by 55% in SW620 cells. KU-0060648 increased the etoposide and doxorubicin cytotoxicity across the DNA-PKcs–proficient cell panel rather than in DNA-PKcs–deficient cells, therefore confirming the enhanced cytotoxicity was due to the inhibition of DNA-PK [1].
In vivo: In mice bearing SW620 and MCF7 xenografts, KU-0060648 concentrations that were sufficient for in vitro growth inhibition and chemosensitization were maintained within the tumor at nontoxic doses for at least 4 hours. KU-0060648 alone delayed the MCF7 xenografts growth and increased etoposide-induced tumor growth delay in both in MCF7 and SW620 xenografts by up to 4.5 folds, without causing etoposide toxicity to unacceptable levels [1].
Clinical trial: KU-0060648 is still in pre-clinical development stage and no clinicl trial is ongoing currently.
Reference:
[1] Munck JM, Batey MA, Zhao Y, Jenkins H, Richardson CJ, Cano C, Tavecchio M, Barbeau J, Bardos J, Cornell L, Griffin RJ, Menear K, Slade A, Thommes P, Martin NM, Newell DR, Smith GC, Curtin NJ. Chemosensitization of cancer cells by KU-0060648, a dual inhibitor of DNA-PK and PI-3K. Mol Cancer Ther. 2012;11(8):1789-98.
- Notoginsenoside Fc
Catalog No.:BCN3853
CAS No.:88122-52-5
- JNJ-26854165 (Serdemetan)
Catalog No.:BCC2240
CAS No.:881202-45-5
- Valganciclovir
Catalog No.:BCC2026
CAS No.:88110-89-8
- Notoginsenoside Fe
Catalog No.:BCN3852
CAS No.:88105-29-7
- 30-Hydroxygambogic acid
Catalog No.:BCN3081
CAS No.:881027-36-7
- HDS 029
Catalog No.:BCC7441
CAS No.:881001-19-0
- Notoginsenoside Fa
Catalog No.:BCN3854
CAS No.:88100-04-3
- Phrixotoxin 3
Catalog No.:BCC6328
CAS No.:880886-00-0
- 4-Hydroxybenzaldehyde rhamnoside
Catalog No.:BCN7625
CAS No.:88086-86-6
- PhiKan 083
Catalog No.:BCC2411
CAS No.:880813-36-5
- Naloxonazine dihydrochloride
Catalog No.:BCC6710
CAS No.:880759-65-9
- AMD-070 hydrochloride
Catalog No.:BCC1358
CAS No.:880549-30-4
- Daphniyunnine A
Catalog No.:BCN4428
CAS No.:881388-87-0
- Daphniyunnine B
Catalog No.:BCN4429
CAS No.:881388-88-1
- 6-Formyllimetin
Catalog No.:BCN3427
CAS No.:88140-31-2
- Dauriporphine
Catalog No.:BCN7903
CAS No.:88142-60-3
- DuP 697
Catalog No.:BCC7064
CAS No.:88149-94-4
- Amlodipine
Catalog No.:BCC4396
CAS No.:88150-42-9
- G-1
Catalog No.:BCC6045
CAS No.:881639-98-1
- 26RFa
Catalog No.:BCC6163
CAS No.:881640-56-8
- Levistilide A
Catalog No.:BCN1197
CAS No.:88182-33-6
- 3,5,9-Trihydroxyergosta-7,22-dien-6-one
Catalog No.:BCN1318
CAS No.:88191-14-4
- MDL 28170
Catalog No.:BCC2352
CAS No.:88191-84-8
- iMDK
Catalog No.:BCC6365
CAS No.:881970-80-5
Chemosensitization of cancer cells by KU-0060648, a dual inhibitor of DNA-PK and PI-3K.[Pubmed:22576130]
Mol Cancer Ther. 2012 Aug;11(8):1789-98.
DNA double-strand breaks (DSB) are the most cytotoxic lesions induced by topoisomerase II poisons. Nonhomologous end joining (NHEJ) is a major pathway for DSB repair and requires DNA-dependent protein kinase (DNA-PK) activity. DNA-PK catalytic subunit (DNA-PKcs) is structurally similar to PI-3K, which promotes cell survival and proliferation and is upregulated in many cancers. KU-0060648 is a dual inhibitor of DNA-PK and PI-3K in vitro. KU-0060648 was investigated in a panel of human breast and colon cancer cells. The compound inhibited cellular DNA-PK autophosphorylation with IC(50) values of 0.019 mumol/L (MCF7 cells) and 0.17 mumol/L (SW620 cells), and PI-3K-mediated AKT phosphorylation with IC(50) values of 0.039 mumol/L (MCF7 cells) and more than 10 mumol/L (SW620 cells). Five-day exposure to 1 mumol/L KU-0060648 inhibited cell proliferation by more than 95% in MCF7 cells but only by 55% in SW620 cells. In clonogenic survival assays, KU-0060648 increased the cytotoxicity of etoposide and doxorubicin across the panel of DNA-PKcs-proficient cells, but not in DNA-PKcs-deficient cells, thus confirming that enhanced cytotoxicity was due to DNA-PK inhibition. In mice bearing SW620 and MCF7 xenografts, concentrations of KU-0060648 that were sufficient for in vitro growth inhibition and chemosensitization were maintained within the tumor for at least 4 hours at nontoxic doses. KU-0060648 alone delayed the growth of MCF7 xenografts and increased etoposide-induced tumor growth delay in both in SW620 and MCF7 xenografts by up to 4.5-fold, without exacerbating etoposide toxicity to unacceptable levels. The proof-of-principle in vitro and in vivo chemosensitization with KU-0060648 justifies further evaluation of dual DNA-PK and PI-3K inhibitors.
KU-0060648 inhibits hepatocellular carcinoma cells through DNA-PKcs-dependent and DNA-PKcs-independent mechanisms.[Pubmed:26933997]
Oncotarget. 2016 Mar 29;7(13):17047-59.
Here we tested anti-tumor activity of KU-0060648 in preclinical hepatocellular carcinoma (HCC) models. Our results demonstrated that KU-0060648 was anti-proliferative and pro-apoptotic in established (HepG2, Huh-7 and KYN-2 lines) and primary human HCC cells, but was non-cytotoxic to non-cancerous HL-7702 hepatocytes. DNA-PKcs (DNA-activated protein kinase catalytic subunit) is an important but not exclusive target of KU-0060648. DNA-PKcs knockdown or dominant negative mutation inhibited HCC cell proliferation. On the other hand, overexpression of wild-type DNA-PKcs enhanced HepG2 cell proliferation. Importantly, KU-0060648 was still cytotoxic to DNA-PKcs-silenced or -mutated HepG2 cells, although its activity in these cells was relatively weak. Further studies showed that KU-0060648 inhibited PI3K-AKT-mTOR activation, independent of DNA-PKcs. Introduction of constitutively-active AKT1 (CA-AKT1) restored AKT-mTOR activation after KU-0060648 treatment in HepG2 cells, and alleviated subsequent cytotoxicity. In vivo, intraperitoneal (i.p.) injection of KU-0060648 significantly inhibited HepG2 xenograft growth in nude mice. AKT-mTOR activation was also inhibited in xenografted tumors. Finally, we showed that DNA-PKcs expression was significantly upregulated in human HCC tissues. Yet miRNA-101, an anti-DNA-PKcs miRNA, was downregulated. Over-expression of miR-101 in HepG2 cells inhibited DNA-PKcs expression and cell proliferation. Together, these results indicate that KU-0060648 inhibits HCC cells through DNA-PKcs-dependent and -independent mechanisms.
Pharmacological inhibition of DNA-PK stimulates Cas9-mediated genome editing.[Pubmed:26307031]
Genome Med. 2015 Aug 27;7:93.
BACKGROUND: The ability to modify the genome of any cell at a precise location has drastically improved with the recent discovery and implementation of CRISPR/Cas9 editing technology. However, the capacity to introduce specific directed changes at given loci is hampered by the fact that the major cellular repair pathway that occurs following Cas9-mediated DNA cleavage is the erroneous non-homologous end joining (NHEJ) pathway. Homology-directed recombination (HDR) is far less efficient than NHEJ and makes screening of clones containing directed changes time-consuming and labor-intensive. METHODS: We investigated the possibility of pharmacologically inhibiting DNA-PKcs, a key player in NHEJ, using small molecule inhibitors (NU7441 and KU-0060648), to ameliorate the rates of HDR repair events. These compounds were tested in a sensitive reporter assay capable of simultaneously informing on NHEJ and HDR, as well as on an endogenous gene targeted by Cas9. RESULTS: We find that NU7441 and KU-0060648 reduce the frequency of NHEJ while increasing the rate of HDR following Cas9-mediated DNA cleavage. CONCLUSIONS: Our results identify two small molecules compatible for use with Cas9-editing technology to improve the frequency of HDR.
Radiosensitising agents for the radiotherapy of cancer: novel molecularly targeted approaches.[Pubmed:19456277]
Expert Opin Ther Pat. 2009 Jun;19(6):775-99.
BACKGROUND: The efficacy of radiotherapy (RT) for cancer treatment is limited by normal tissue toxicity and by the intrinsic or acquired radioresistance of many tumours. Therefore, continuing efforts are conducted to identify radiosensitising agents that preferentially sensitise tumour cells to the cytotoxic action of RT. Recent progresses in molecular oncology have uncovered an array of novel targets, which may be exploited for RT enhancement. OBJECTIVE: To survey the patent literature of the past 4 years pertaining to the development of molecularly targeted agents as potential tumour radiosensitisers. METHODS: Patents were searched with a set of relevant keywords using several search engines. A Medline search on the same topics was performed in parallel. RESULTS/CONCLUSION: A total of 48 patents/applications were selected. These concerned agents target molecular components of pathways involved in DNA damage repair, cell growth and survival signalling, apoptosis modulation and tumour angiogenesis. Current trials of some of these agents may reveal their value as clinical radiosensitisers.


