Naloxonazine dihydrochlorideCAS# 880759-65-9 |
- Leupeptin, Microbial
Catalog No.:BCC1217
CAS No.:103476-89-7
- BCX 1470
Catalog No.:BCC1413
CAS No.:217099-43-9
- BCX 1470 methanesulfonate
Catalog No.:BCC1414
CAS No.:217099-44-0
- PMSF
Catalog No.:BCC1229
CAS No.:329-98-6
- Nafamostat hydrochloride
Catalog No.:BCC4188
CAS No.:80251-32-7
- Nafamostat
Catalog No.:BCC4187
CAS No.:81525-10-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 880759-65-9 | SDF | Download SDF |
| PubChem ID | 90479735 | Appearance | Powder |
| Formula | C38H44Cl2N4O6 | M.Wt | 723.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
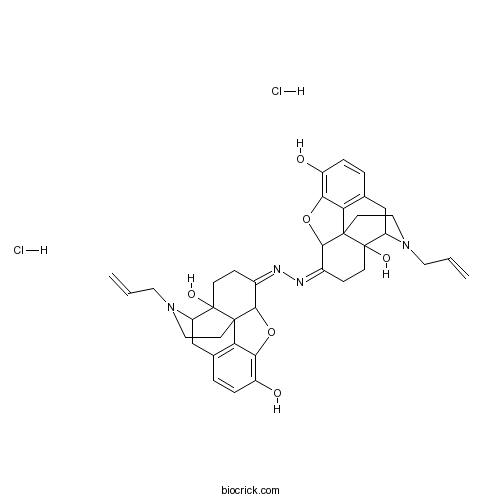
| Chemical Name | (7Z)-7-[(Z)-(4a,9-dihydroxy-3-prop-2-enyl-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinolin-7-ylidene)hydrazinylidene]-3-prop-2-enyl-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-4a,9-diol;dihydrochloride | ||
| SMILES | C=CCN1CCC23C4C(=NN=C5CCC6(C7CC8=C9C6(C5OC9=C(C=C8)O)CCN7CC=C)O)CCC2(C1CC1=C3C(=C(C=C1)O)O4)O.Cl.Cl | ||
| Standard InChIKey | VIAIHLLKDJKEKM-JNHLASQBSA-N | ||
| Standard InChI | InChI=1S/C38H42N4O6.2ClH/c1-3-15-41-17-13-35-29-21-5-7-25(43)31(29)47-33(35)23(9-11-37(35,45)27(41)19-21)39-40-24-10-12-38(46)28-20-22-6-8-26(44)32-30(22)36(38,34(24)48-32)14-18-42(28)16-4-2;;/h3-8,27-28,33-34,43-46H,1-2,9-20H2;2*1H/b39-23-,40-24-;; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent opioid antagonist, selective for μ1 receptors. |

Naloxonazine dihydrochloride Dilution Calculator

Naloxonazine dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3818 mL | 6.909 mL | 13.8181 mL | 27.6361 mL | 34.5452 mL |
| 5 mM | 0.2764 mL | 1.3818 mL | 2.7636 mL | 5.5272 mL | 6.909 mL |
| 10 mM | 0.1382 mL | 0.6909 mL | 1.3818 mL | 2.7636 mL | 3.4545 mL |
| 50 mM | 0.0276 mL | 0.1382 mL | 0.2764 mL | 0.5527 mL | 0.6909 mL |
| 100 mM | 0.0138 mL | 0.0691 mL | 0.1382 mL | 0.2764 mL | 0.3455 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AMD-070 hydrochloride
Catalog No.:BCC1358
CAS No.:880549-30-4
- Fmoc-Hyp-OH
Catalog No.:BCC3254
CAS No.:88050-17-3
- Neuropathiazol
Catalog No.:BCC5375
CAS No.:880090-88-0
- 1,2-Benzenedicarboxylic acid
Catalog No.:BCN3151
CAS No.:88-99-3
- BHQ
Catalog No.:BCC6982
CAS No.:88-58-4
- 2-Acetylthiophene
Catalog No.:BCC8517
CAS No.:88-15-3
- Furan-2-carboxylic acid
Catalog No.:BCN4557
CAS No.:88-14-2
- Ingenol-5,20-acetonide-3-O-angelate
Catalog No.:BCN8458
CAS No.:87980-68-5
- UFP 803
Catalog No.:BCC5917
CAS No.:879497-82-2
- Prionoid E
Catalog No.:BCN3161
CAS No.:879324-78-4
- Prionoid D
Catalog No.:BCN3160
CAS No.:879324-77-3
- Prionoid C
Catalog No.:BCN3159
CAS No.:879324-76-2
- PhiKan 083
Catalog No.:BCC2411
CAS No.:880813-36-5
- 4-Hydroxybenzaldehyde rhamnoside
Catalog No.:BCN7625
CAS No.:88086-86-6
- Phrixotoxin 3
Catalog No.:BCC6328
CAS No.:880886-00-0
- Notoginsenoside Fa
Catalog No.:BCN3854
CAS No.:88100-04-3
- HDS 029
Catalog No.:BCC7441
CAS No.:881001-19-0
- 30-Hydroxygambogic acid
Catalog No.:BCN3081
CAS No.:881027-36-7
- Notoginsenoside Fe
Catalog No.:BCN3852
CAS No.:88105-29-7
- Valganciclovir
Catalog No.:BCC2026
CAS No.:88110-89-8
- JNJ-26854165 (Serdemetan)
Catalog No.:BCC2240
CAS No.:881202-45-5
- Notoginsenoside Fc
Catalog No.:BCN3853
CAS No.:88122-52-5
- KU-0060648
Catalog No.:BCC1110
CAS No.:881375-00-4
- Daphniyunnine A
Catalog No.:BCN4428
CAS No.:881388-87-0
Inhibition of morphine-induced cAMP overshoot: a cell-based assay model in a high-throughput format.[Pubmed:21598037]
Cell Mol Neurobiol. 2011 Aug;31(6):901-7.
Opiates are not only potent analgesics but also drugs of abuse mainly because they produce euphoria. Chronic use of opiates results in the development of tolerance and dependence. Dr Marshall Nirenberg's group at the National Institutes of Health (NIH) was the first to use a cellular model system of Neuroblastoma x Glioma hybrid cells (NG108-15) to study morphine addiction. They showed that opiates affect adenylyl cyclase (AC) by two opposing mechanisms mediated by the opiate receptor. Although the cellular mechanisms that cause addiction are not yet completely understood, the most observed correlative biochemical adaptation is the upregulation of AC. This model also provides the opportunity to look for compounds which could dissociate the acute effect of opiates from the delayed response, upregulation of AC, and thus lead to the discovery of non-addictive drugs. To identify small molecule compounds that can inhibit morphine-induced cAMP overshoot, we have validated and optimized a cell-based assay in a high throughput format that measures cellular cAMP production after morphine withdrawal. The assay performed well in the 1536-well plate format. The LOPAC library of 1,280 compounds was screened in this assay on a quantitative high-throughput screening (qHTS) platform. A group of compounds that can inhibit morphine-induced cAMP overshoot were identified. The most potent compounds are eight naloxone-related compounds, including levallorphan tartrate, Naloxonazine dihydrochloride, naloxone hydrochloride, naltrexone hydrochloride, and naltriben methanesulfonate. The qHTS approach we used in this study will be useful in identifying novel inhibitors of morphine induced addiction from a larger scale screening.
Structural determination of the novel fragmentation routes of zwitteronic morphine opiate antagonists naloxonazine and naloxone hydrochlorides using electrospray ionization tandem mass spectrometry.[Pubmed:17310471]
Rapid Commun Mass Spectrom. 2007;21(6):1062-74.
Electrospray ionization quadrupole time-of-flight (ESI-QqToF) mass spectra of the zwitteronic salts Naloxonazine dihydrochloride 1 and naloxone hydrochloride 2, a common series of morphine opiate receptor antagonists, were recorded using different declustering potentials. The singly charged ion [M+H-2HCl](+) at m/z 651.3170 and the doubly charged ion [M+2H-2HCl](2+) at m/z 326.1700 were noted for Naloxonazine dihydrochloride 1; and the singly charged ion [M+H-HCl](+) at m/z 328.1541 was observed for naloxone hydrochloride 2. Low-energy collision-induced dissociation tandem mass spectrometry (CID-MS/MS) experiments established the fragmentation routes of these compounds. In addition to the characteristic diagnostic product ions obtained, we noticed the formation of a series of radical product ions for the zwitteronic compounds 1 and 2, and also the formation of a distonic ion product formed from the singly charged ion [M+H-HCl](+) of naloxone hydrochloride 2. Confirmation of the various established fragmentation routes was effected by conducting a series of ESI-CID-QqTof-MS/MS product ion scans, which were initiated by CID in the atmospheric pressure/vacuum interface using a higher declustering potential. Deuterium labeling was also performed on the zwitteronic salts 1 and 2, in which the hydrogen atoms of the OH and NH groups were exchanged with deuterium atoms. Low-energy CID-QqTof-MS/MS product ion scans of the singly charged and doubly charged deuteriated molecules confirmed the initial fragmentation patterns proposed for the protonated molecules. Precursor ion scan analyses were also performed with a conventional quadrupole-hexapole-quadrupole tandem mass spectrometer and allowed the confirmation of the genesis of some diagnostic ions.


