Levistilide ACAS# 88182-33-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 88182-33-6 | SDF | Download SDF |
| PubChem ID | 70698035 | Appearance | Cryst. |
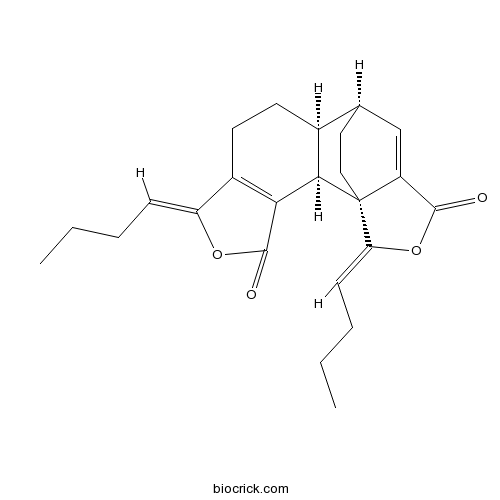
| Formula | C24H28O4 | M.Wt | 380.48 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 142797-35-1;Senkyunolide O | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CCCC=C1C2=C(C3C(CC2)C4CCC35C(=C4)C(=O)OC5=CCCC)C(=O)O1 | ||
| Standard InChIKey | UBBRXVRQZJSDAK-ZJHGLIIDSA-N | ||
| Standard InChI | InChI=1S/C24H28O4/c1-3-5-7-18-16-10-9-15-14-11-12-24(21(15)20(16)23(26)27-18)17(13-14)22(25)28-19(24)8-6-4-2/h7-8,13-15,21H,3-6,9-12H2,1-2H3/b18-7-,19-8-/t14-,15+,21-,24+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Traditional formula compatibility of Danggui-Shaoyao-San could significantly enhance levistilide A bioavailability compared with levistilide A alone and Rhizoma Chuanxiong. Senkyunolide O is a COX-2 selective inhibitor with the IC(50) value of 5 microM. |
| Targets | COX |
| Structure Identification | J Chromatogr Sci. 2015 Feb 5. pii: bmu224.Study on Pharmacokinetics of Three Preparations from Levistolide A by LC-MS-MS.[Pubmed: 25657289]A rapid sensitive analytical method was established and validated to investigate levistolide A in rat plasma by liquid chromatography-tandem mass spectrometry operated in the positive ion mode.
|

Levistilide A Dilution Calculator

Levistilide A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6283 mL | 13.1413 mL | 26.2826 mL | 52.5652 mL | 65.7065 mL |
| 5 mM | 0.5257 mL | 2.6283 mL | 5.2565 mL | 10.513 mL | 13.1413 mL |
| 10 mM | 0.2628 mL | 1.3141 mL | 2.6283 mL | 5.2565 mL | 6.5706 mL |
| 50 mM | 0.0526 mL | 0.2628 mL | 0.5257 mL | 1.0513 mL | 1.3141 mL |
| 100 mM | 0.0263 mL | 0.1314 mL | 0.2628 mL | 0.5257 mL | 0.6571 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 26RFa
Catalog No.:BCC6163
CAS No.:881640-56-8
- G-1
Catalog No.:BCC6045
CAS No.:881639-98-1
- Amlodipine
Catalog No.:BCC4396
CAS No.:88150-42-9
- DuP 697
Catalog No.:BCC7064
CAS No.:88149-94-4
- Dauriporphine
Catalog No.:BCN7903
CAS No.:88142-60-3
- 6-Formyllimetin
Catalog No.:BCN3427
CAS No.:88140-31-2
- Daphniyunnine B
Catalog No.:BCN4429
CAS No.:881388-88-1
- Daphniyunnine A
Catalog No.:BCN4428
CAS No.:881388-87-0
- KU-0060648
Catalog No.:BCC1110
CAS No.:881375-00-4
- Notoginsenoside Fc
Catalog No.:BCN3853
CAS No.:88122-52-5
- JNJ-26854165 (Serdemetan)
Catalog No.:BCC2240
CAS No.:881202-45-5
- Valganciclovir
Catalog No.:BCC2026
CAS No.:88110-89-8
- 3,5,9-Trihydroxyergosta-7,22-dien-6-one
Catalog No.:BCN1318
CAS No.:88191-14-4
- MDL 28170
Catalog No.:BCC2352
CAS No.:88191-84-8
- iMDK
Catalog No.:BCC6365
CAS No.:881970-80-5
- Clofibric Acid
Catalog No.:BCC4652
CAS No.:882-09-7
- (-)-Chelidonine
Catalog No.:BCN7456
CAS No.:88200-01-5
- Notopterol
Catalog No.:BCN5386
CAS No.:88206-46-6
- H-Leu-OAll.TosOH
Catalog No.:BCC2969
CAS No.:88224-03-7
- H-Ile-OAll.TosOH
Catalog No.:BCC2963
CAS No.:88224-05-9
- P005091
Catalog No.:BCC1287
CAS No.:882257-11-6
- AI-3
Catalog No.:BCC8018
CAS No.:882288-28-0
- R1530
Catalog No.:BCC1879
CAS No.:882531-87-5
- Netobimin
Catalog No.:BCC9100
CAS No.:88255-01-0
[Study on biligustilides from Angelica sinensis].[Pubmed:19166005]
Zhongguo Zhong Yao Za Zhi. 2008 Oct;33(19):2196-201.
OBJECTIVE: To study the chemical constituents of Angelica sinensis. METHOD: The constituents were separated by chromatographic methods, and their structures were identified on the basis of spectroscopic analysis. RESULT: Eight compounds were isolated and identified as levistolide A (1), senkyunolide O (2), (3Z, 3Z')-6.8', 7.3'-diligustilide (3), tokinolide B (4), isotokinolide B (5), (3'Z)-(3R, 8S, 3a'R, 6'S)-3, 3a': 8, 6'-biligustilide (6), E, E'-3. 3', 8. 8'-diligustilide (7) and E, E'-3. 3', 8. 8'-isodiligustilide (8), which are all diligustilides. CONCLUSION: Compound 7 was obtained from the plant for the first time; compounds 6 and 8 are new compounds.
Discovery of cyclooxygenase inhibitors from medicinal plants used to treat inflammation.[Pubmed:20188172]
Pharmacol Res. 2010 Jun;61(6):519-24.
Eleven authenticated botanicals used in the traditional Chinese medicine Huo-Luo-Xiao-Ling Dan were screened for ligands to cyclooxygenase (COX) using pulsed ultrafiltration liquid chromatography-mass spectrometry, and a mass spectrometry-based enzyme assay was used to determine the concentration of each of 17 ligands that inhibited COX-1 or COX-2 by 50% (IC(50)). Acetyl-11-keto-beta-boswellic acid, beta-boswellic acid, acetyl-alpha-boswellic acid, acetyl-beta-boswellic acid, and betulinic acid were COX-1 selective inhibitors with IC(50) values of approximately 10 microM. Senkyunolide O and cryptotanshinone were COX-2 selective inhibitors with IC(50) values of 5 microM and 22 microM, respectively. Roburic acid and phenethyl-trans-ferulate inhibited COX-1 and COX-2 equally. COX inhibition and the IC(50) values of most of these natural product ligands have not been reported previously.
Study on Pharmacokinetics of Three Preparations from Levistolide A by LC-MS-MS.[Pubmed:25657289]
J Chromatogr Sci. 2015 Sep;53(8):1265-73.
A rapid sensitive analytical method was established and validated to investigate levistolide A in rat plasma by liquid chromatography-tandem mass spectrometry operated in the positive ion mode. Levistilide A (LA) and internal standard (IS) andrographolide (AD), mixed with the plasma sample, were separated on a reversed phase Spursil C18 5 microm column. The precursor/product transitions (m/z) were 398.5/381.3 for LA and (m/z) 368.0/351.1 for AD. The calibration curve was linear over the range from 5 to 1,250 ng/mL for oral administration and 10-4,000 for intravenous administration with a correlation coefficient (r) >/=0.9993. The lower limit of quantification was 5 ng/mL for LA in plasma. The inter- and intra-day accuracy and precision were less than +/-15% of the relative standard deviation. In this study, the developed method is successfully applied to the comparative pharmacokinetic study of LA in rats after oral administration of LA alone, Rhizoma Chuanxiong, and Danggui-Shaoyao-San along with the bioavailability study of LA in rats. Our study shows that low bioavailability (7.5%) is observed after oral administration of LA. Traditional formula compatibility of Danggui-Shaoyao-San could significantly enhance LA bioavailability compared with LA alone and Rhizoma Chuanxiong.


