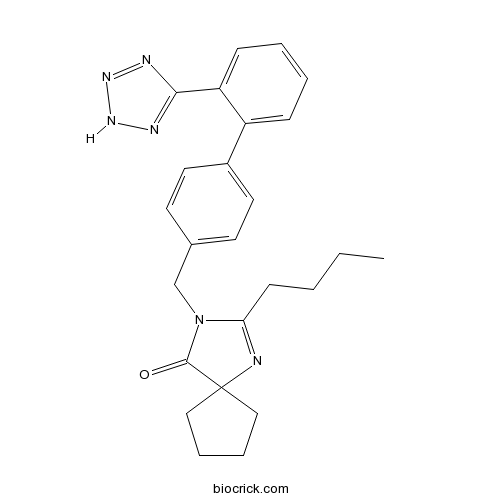
IrbesartanAngiotensin II inhibitor CAS# 138402-11-6 |
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- Olmesartan
Catalog No.:BCC1819
CAS No.:144689-24-7
- AVE 0991
Catalog No.:BCC4032
CAS No.:304462-19-9
- Tranilast
Catalog No.:BCC2514
CAS No.:53902-12-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 138402-11-6 | SDF | Download SDF |
| PubChem ID | 3749 | Appearance | Powder |
| Formula | C25H28N6O | M.Wt | 428.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SR-47436; BMS-186295 | ||
| Solubility | DMSO : 100 mg/mL (233.36 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 2-butyl-3-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]methyl]-1,3-diazaspiro[4.4]non-1-en-4-one | ||
| SMILES | CCCCC1=NC2(CCCC2)C(=O)N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NNN=N5 | ||
| Standard InChIKey | YOSHYTLCDANDAN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent angiotensin II type 1 receptor (AT1) antagonist (IC50 = 1.3 nM). Displays antihypertensive activity. Orally bioavailable. |

Irbesartan Dilution Calculator

Irbesartan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3336 mL | 11.6678 mL | 23.3356 mL | 46.6712 mL | 58.339 mL |
| 5 mM | 0.4667 mL | 2.3336 mL | 4.6671 mL | 9.3342 mL | 11.6678 mL |
| 10 mM | 0.2334 mL | 1.1668 mL | 2.3336 mL | 4.6671 mL | 5.8339 mL |
| 50 mM | 0.0467 mL | 0.2334 mL | 0.4667 mL | 0.9334 mL | 1.1668 mL |
| 100 mM | 0.0233 mL | 0.1167 mL | 0.2334 mL | 0.4667 mL | 0.5834 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Irbesartan (Avapro) belongs to the class of medicines called angiotensin II inhibitor antihypertensives. It is used to treat high blood pressure(hypertension). Irbesartan works by blocking the action of a substance in the body that causes blood vessels to
- Tinospinoside C
Catalog No.:BCN6925
CAS No.:1383977-51-2
- Afzelechin-(4alpha->8)-epiafzelechin
Catalog No.:BCN7709
CAS No.:1383627-30-2
- Boc-Arg(Tos)-OH
Catalog No.:BCC3067
CAS No.:13836-37-8
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- BD 1047 dihydrobromide
Catalog No.:BCC6863
CAS No.:138356-21-5
- BD 1008 dihydrobromide
Catalog No.:BCC6674
CAS No.:138356-09-9
- CYM 9484
Catalog No.:BCC6238
CAS No.:1383478-94-1
- Hedycoronen A
Catalog No.:BCN7653
CAS No.:1383441-73-3
- Stearyl glycyrrhetinate
Catalog No.:BCN8486
CAS No.:13832-70-7
- ML 289
Catalog No.:BCC6343
CAS No.:1382481-79-9
- (RS)-(Tetrazol-5-yl)glycine
Catalog No.:BCC6599
CAS No.:138199-51-6
- H-β-HoPhe-OH
Catalog No.:BCC3240
CAS No.:138165-77-2
- A 1070722
Catalog No.:BCC7933
CAS No.:1384424-80-9
- FK 888
Catalog No.:BCC5907
CAS No.:138449-07-7
- Ro 31-8220 Mesylate
Catalog No.:BCC4999
CAS No.:138489-18-6
- S-(+)-Marmesin
Catalog No.:BCN8288
CAS No.:13849-08-6
- 3-Oxopomolic acid
Catalog No.:BCN3485
CAS No.:13849-90-6
- Pomolic acid
Catalog No.:BCN6197
CAS No.:13849-91-7
- Tormentic acid
Catalog No.:BCN6198
CAS No.:13850-16-3
- 9-Hydroxycanthin-6-one
Catalog No.:BCN3105
CAS No.:138544-91-9
- N3PT
Catalog No.:BCC1782
CAS No.:13860-66-7
- NKH 477
Catalog No.:BCC7126
CAS No.:138605-00-2
- (R)-3-Carboxy-4-hydroxyphenylglycine
Catalog No.:BCC6606
CAS No.:13861-03-5
- Securitinine
Catalog No.:BCN6986
CAS No.:13861-71-7
Cardioprotective effects of irbesartan in polymicrobial sepsis : The role of the p38MAPK/NF-kappaB signaling pathway.[Pubmed:28144715]
Herz. 2018 Mar;43(2):140-145.
BACKGROUND: Sepsis is a systemic inflammatory response usually correlated with multi-organ failure. Myocardial dysfunction is one of the adverse outcomes in septic patients and results in high mortality rates. The aim of this study was to investigate the impact of Irbesartan in attenuation of cardiac depression during polymicrobial sepsis via decreased activation of the phospho-p38MAPK/nuclear factor (NF)-kappaB signaling pathway. MATERIALS AND METHODS: A model of polymicrobial sepsis induced via cecal ligation and puncture (CLP) with 8- to 12-week-old albino mice was used. Mice were treated with i.p. Irbesartan (3 mg/kg) 1 h before CLP. Using a micro-tipped transducer catheter, the following hemodynamic parameters were evaluated after CLP: heart rate, ejection fraction, left ventricular (LV) end-diastolic pressure, LV systolic pressure, and cardiac output. Plasma levels of proinflammatory cytokines, including tumor necrosis factor (TNF)-alpha, interleukin (IL)-1 beta, IL-6, monocyte chemoattractant protein-1 (MCP-1), and cardiac troponin I (cTn-I), were measured via ELISA analysis. The degree of p38MAPK and NF-kappaB phosphorylation was assessed via Western blotting. RESULTS: Mice treated with Irbesartan displayed improvement in LV function (ejection fraction: 42.4 +/- 1.1% vs. 27.8 +/- 3% in CLP mice). The attenuation of cardiac depression in Irbesartan-treated mice was associated with lower levels of MCP-1 in plasma and a reduction in the levels of TNF-alpha, IL-1beta, and IL-6. Furthermore, Irbesartan-treated mice displayed lower expression levels of p38-MAPK and NF-kappaB phosphorylation. CONCLUSION: Irbesartan can attenuate cardiac dysfunction during polymicrobial sepsis possibly via a reduction of proinflammatory cytokines through decreased activation of the p38MAPK/NF-kappaB pathways.
Exploring the interactions of irbesartan and irbesartan-2-hydroxypropyl-beta-cyclodextrin complex with model membranes.[Pubmed:28274845]
Biochim Biophys Acta Biomembr. 2017 Jun;1859(6):1089-1098.
The interactions of Irbesartan (IRB) and Irbesartan-2-hydroxypropyl-beta-cyclodextrin (HP-beta-CD) complex with dipalmitoyl phosphatidylcholine (DPPC) bilayers have been explored utilizing an array of biophysical techniques ranging from differential scanning calorimetry (DSC), small angle X-ray scattering (SAXS), ESI mass spectrometry (ESI-MS) and solid state nuclear magnetic resonance (ssNMR). Molecular dynamics (MD) calculations have been also conducted to complement the experimental results. Irbesartan was found to be embedded in the lipid membrane core and to affect the phase transition properties of the DPPC bilayers. SAXS studies revealed that Irbesartan alone does not display perfect solvation since some coexisting Irbesartan crystallites are present. In its complexed form IRB gets fully solvated in the membranes showing that encapsulation of IRB in HP-beta-CD may have beneficial effects in the ADME properties of this drug. MD experiments revealed the topological and orientational integration of Irbesartan into the phospholipid bilayer being placed at about 1nm from the membrane centre.
Efficacy of Zofenopril vs. Irbesartan in Combination with a Thiazide Diuretic in Hypertensive Patients with Multiple Risk Factors not Controlled by a Previous Monotherapy: A Review of the Double-Blind, Randomized "Z" Studies.[Pubmed:28260186]
Adv Ther. 2017 Apr;34(4):784-798.
Combinations between an angiotensin converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB) and hydrochlorothiazide (HCTZ) are among the recommended treatments for hypertensive patients uncontrolled by monotherapy. Four randomized, double-blind, parallel group studies with a similar design, including 1469 hypertensive patients uncontrolled by a previous monotherapy and with >/=1 cardiovascular risk factor, compared the efficacy of a combination of a sulfhydryl ACE inhibitor (zofenopril at 30 or 60 mg) or an ARB (Irbesartan at 150 or 300 mg) plus HCTZ 12.5 mg. The extent of blood pressure (BP)-lowering was assessed in the office and over 24 h. Pleiotropic features of the treatments were evaluated by studying their effect on systemic inflammation, organ damage, arterial stiffness, and metabolic biochemical parameters. Both treatments similarly reduced office and ambulatory BPs after 18-24 weeks. In the ZODIAC study a larger reduction in high sensitivity C reactive protein (hs-CRP) was observed under zofenopril (-0.52 vs. +0.97 mg/dL under Irbesartan, p = 0.001), suggesting a potential protective effect against the development of atherosclerosis. In the ZENITH study the rate of carotid plaque regression was significantly larger under zofenopril (32% vs. 16%; p = 0.047). In the diabetic patients of the ZAMES study, no adverse effects of treatments on blood glucose and lipids as well as an improvement of renal function were observed. In patients with isolated systolic hypertension of the ZEUS study, a slight and similar improvement in renal function and small reductions in pulse wave velocity (PWV), augmentation index (AI), and central systolic BP were documented with both treatments. Thus, the fixed combination of zofenopril and HCTZ may have a relevant place in the treatment of high-risk or monotherapy-treated uncontrolled hypertensive patients requiring a more prompt, intensive, and sustained BP reduction, in line with the recommendations of current guidelines.
Clinical and Echocardiographic Characteristics and Cardiovascular Outcomes According to Diabetes Status in Patients With Heart Failure and Preserved Ejection Fraction: A Report From the I-Preserve Trial (Irbesartan in Heart Failure With Preserved Ejection Fraction).[Pubmed:28052977]
Circulation. 2017 Feb 21;135(8):724-735.
BACKGROUND: In patients with heart failure and preserved ejection fraction, little is known about the characteristics of, and outcomes in, those with and without diabetes mellitus. METHODS: We examined clinical and echocardiographic characteristics and outcomes in the I-Preserve trial (Irbesartan in Heart Failure With Preserved Ejection Fraction) according to history of diabetes mellitus. Cox regression models were used to estimate hazard ratios for cardiovascular outcomes adjusted for known predictors, including age, sex, natriuretic peptides, and comorbidity. Echocardiographic data were available in 745 patients and were additionally adjusted for in supplementary analyses. RESULTS: Overall, 1134 of 4128 patients (27%) had diabetes mellitus. Compared with those without diabetes mellitus, they were more likely to have a history of myocardial infarction (28% versus 22%), higher body mass index (31 versus 29 kg/m(2)), worse Minnesota Living With Heart Failure score (48 versus 40), higher median N-terminal pro-B-type natriuretic peptide concentration (403 versus 320 pg/mL; all P<0.01), more signs of congestion, but no significant difference in left ventricular ejection fraction. Patients with diabetes mellitus had a greater left ventricular mass and left atrial area than patients without diabetes mellitus. Doppler E-wave velocity (86 versus 76 cm/s; P<0.0001) and the E/e' ratio (11.7 versus 10.4; P=0.010) were higher in patients with diabetes mellitus. Over a median follow-up of 4.1 years, cardiovascular death or heart failure hospitalization occurred in 34% of patients with diabetes mellitus versus 22% of those without diabetes mellitus (adjusted hazard ratio, 1.75; 95% confidence interval, 1.49-2.05), and 28% versus 19% of patients with and without diabetes mellitus died (adjusted hazard ratio, 1.59; confidence interval, 1.33-1.91). CONCLUSIONS: In heart failure with preserved ejection fraction, patients with diabetes mellitus have more signs of congestion, worse quality of life, higher N-terminal pro-B-type natriuretic peptide levels, and a poorer prognosis. They also display greater structural and functional echocardiographic abnormalities. Further investigation is needed to determine the mediators of the adverse impact of diabetes mellitus on outcomes in heart failure with preserved ejection fraction and whether they are modifiable. CLINICAL TRIAL REGISTRATION: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00095238.


