Hedycoronen ACAS# 1383441-73-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1383441-73-3 | SDF | Download SDF |
| PubChem ID | 56931827 | Appearance | Powder |
| Formula | C21H30O3 | M.Wt | 330.46 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
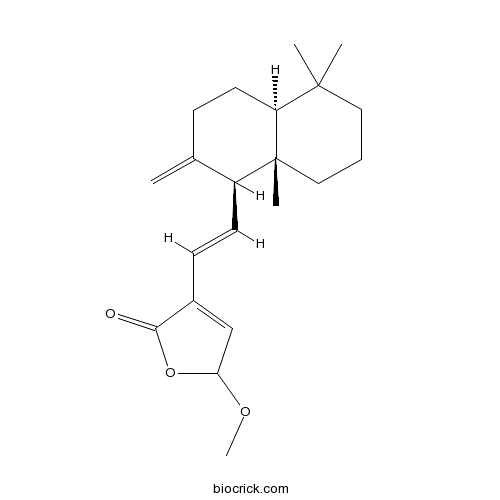
| Chemical Name | 4-[(E)-2-[(1S,4aS,8aS)-5,5,8a-trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]ethenyl]-2-methoxy-2H-furan-5-one | ||
| SMILES | CC1(CCCC2(C1CCC(=C)C2C=CC3=CC(OC3=O)OC)C)C | ||
| Standard InChIKey | BQAWJLFWEBGZIH-MZBNRDNVSA-N | ||
| Standard InChI | InChI=1S/C21H30O3/c1-14-7-10-17-20(2,3)11-6-12-21(17,4)16(14)9-8-15-13-18(23-5)24-19(15)22/h8-9,13,16-18H,1,6-7,10-12H2,2-5H3/b9-8+/t16-,17-,18?,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Hedycoronen A and hedycoronen B are potent inhibitors of LPS-stimulated interleukin-6 (IL-6) and IL-12 p40, with IC(50) ranging from 4.1±0.2 to 9.1±0.3 uM, they also show moderate inhibitory activity on the tumor necrosis factor-α (TNF-α) production with IC(50) values of 46.0±1.3 and 12.7±0.3 uM, suggests that they have the potential anti-inflammatory benefits. |
| Targets | TNF-α | IL Receptor |

Hedycoronen A Dilution Calculator

Hedycoronen A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0261 mL | 15.1304 mL | 30.2608 mL | 60.5217 mL | 75.6521 mL |
| 5 mM | 0.6052 mL | 3.0261 mL | 6.0522 mL | 12.1043 mL | 15.1304 mL |
| 10 mM | 0.3026 mL | 1.513 mL | 3.0261 mL | 6.0522 mL | 7.5652 mL |
| 50 mM | 0.0605 mL | 0.3026 mL | 0.6052 mL | 1.2104 mL | 1.513 mL |
| 100 mM | 0.0303 mL | 0.1513 mL | 0.3026 mL | 0.6052 mL | 0.7565 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Stearyl glycyrrhetinate
Catalog No.:BCN8486
CAS No.:13832-70-7
- ML 289
Catalog No.:BCC6343
CAS No.:1382481-79-9
- (RS)-(Tetrazol-5-yl)glycine
Catalog No.:BCC6599
CAS No.:138199-51-6
- H-β-HoPhe-OH
Catalog No.:BCC3240
CAS No.:138165-77-2
- 7-Methoxy-1-naphthylacetonitrile
Catalog No.:BCN2242
CAS No.:138113-08-3
- Agomelatine
Catalog No.:BCN2165
CAS No.:138112-76-2
- (R,R)-THC
Catalog No.:BCC7224
CAS No.:138090-06-9
- Acetylanonamine
Catalog No.:BCN2140
CAS No.:138079-62-6
- G007-LK
Catalog No.:BCC6383
CAS No.:1380672-07-0
- LDK378 dihydrochloride
Catalog No.:BCC1694
CAS No.:1380575-43-8
- 2-(4-Hydroxy-2-oxoindolin-3-yl)acetonitrile
Catalog No.:BCN1575
CAS No.:1380540-77-1
- YM 750
Catalog No.:BCC7542
CAS No.:138046-43-2
- CYM 9484
Catalog No.:BCC6238
CAS No.:1383478-94-1
- BD 1008 dihydrobromide
Catalog No.:BCC6674
CAS No.:138356-09-9
- BD 1047 dihydrobromide
Catalog No.:BCC6863
CAS No.:138356-21-5
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Boc-Arg(Tos)-OH
Catalog No.:BCC3067
CAS No.:13836-37-8
- Afzelechin-(4alpha->8)-epiafzelechin
Catalog No.:BCN7709
CAS No.:1383627-30-2
- Tinospinoside C
Catalog No.:BCN6925
CAS No.:1383977-51-2
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- A 1070722
Catalog No.:BCC7933
CAS No.:1384424-80-9
- FK 888
Catalog No.:BCC5907
CAS No.:138449-07-7
- Ro 31-8220 Mesylate
Catalog No.:BCC4999
CAS No.:138489-18-6
- S-(+)-Marmesin
Catalog No.:BCN8288
CAS No.:13849-08-6
Labdane-type diterpenoids from the rhizomes of Hedychium coronarium inhibit lipopolysaccharide-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells.[Pubmed:22293485]
Chem Pharm Bull (Tokyo). 2012;60(2):246-50.
The rhizomes of Hedychium coronarium have been used for the treatment of inflammation, skin diseases, headache, and sharp pain due to rheumatism in traditional medicine. From this plant, two new labdanes, 15-methoxylabda-8(17),11E,13-trien-16,15-olide (1) and 16-methoxylabda-8(17),11E,13-trien-15,16-olide (3), named hedycoronens A and B, as well as four known, labda-8(17),11,13-trien-16,15-olide (2), 16-hydroxylabda-8(17),11,13-trien-15,16-olide (4), coronarin A (5), and corronarin E (6) were isolated. Their chemical structures were elucidated by mass, 1D- and 2D-nuclear magnetic resonance (NMR) spectroscopy. They were evaluated for inhibitory effects on the lipopolysaccharide (LPS)-stimulated production of pro-inflammatory cytokines in bone marrow-derived dendritic cells. Among of them, compounds 1-3 were potent inhibitors of LPS-stimulated interleukin-6 (IL-6) and IL-12 p40, with IC(50) ranging from 4.1+/-0.2 to 9.1+/-0.3 muM. Compounds 1 and 3 showed moderate inhibitory activity on the tumor necrosis factor-alpha (TNF-alpha) production with IC(50) values of 46.0+/-1.3 and 12.7+/-0.3 muM. The remains of compounds showed inactivity. These results warrant further studies concerning the potential anti-inflammatory benefits of labdane-diterpenes from H. coronarium.


