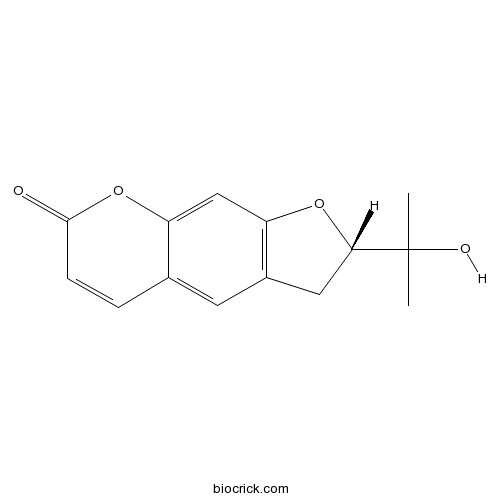
S-(+)-MarmesinCAS# 13849-08-6 |
- (±)-Marmesin
Catalog No.:BCN3618
CAS No.:13710-70-8
- Nodakenetin
Catalog No.:BCN5604
CAS No.:495-32-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13849-08-6 | SDF | Download SDF |
| PubChem ID | 334704 | Appearance | White powder |
| Formula | C14H14O4 | M.Wt | 246.26 |
| Type of Compound | Phenylpropanes | Storage | Desiccate at -20°C |
| Synonyms | (+)-Marmesin; (S)-Marmesin | ||
| Solubility | DMSO : 2 mg/mL (8.12 mM; Need ultrasonic) | ||
| Chemical Name | (2S)-2-(2-hydroxypropan-2-yl)-2,3-dihydrofuro[3,2-g]chromen-7-one | ||
| SMILES | CC(C)(C1CC2=C(O1)C=C3C(=C2)C=CC(=O)O3)O | ||
| Standard InChIKey | FWYSBEAFFPBAQU-LBPRGKRZSA-N | ||
| Standard InChI | InChI=1S/C14H14O4/c1-14(2,16)12-6-9-5-8-3-4-13(15)18-10(8)7-11(9)17-12/h3-5,7,12,16H,6H2,1-2H3/t12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

S-(+)-Marmesin Dilution Calculator

S-(+)-Marmesin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0607 mL | 20.3037 mL | 40.6075 mL | 81.215 mL | 101.5187 mL |
| 5 mM | 0.8121 mL | 4.0607 mL | 8.1215 mL | 16.243 mL | 20.3037 mL |
| 10 mM | 0.4061 mL | 2.0304 mL | 4.0607 mL | 8.1215 mL | 10.1519 mL |
| 50 mM | 0.0812 mL | 0.4061 mL | 0.8121 mL | 1.6243 mL | 2.0304 mL |
| 100 mM | 0.0406 mL | 0.203 mL | 0.4061 mL | 0.8121 mL | 1.0152 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
S-(+)-Marmesin is a natural coumarin, exhibiting COX-2/5-LOX dual inhibitory activity.
In Vitro:S-(+)-Marmesin ((+)-marmesin) shows affinity at the recombinant psoralen synthase, with a Km of 1.5 ± 0.5 μM, exceeding the substrate affinities of other enzymes of the CYP71 subfamily involved in plant secondary metabolism[1]. S-(+)-Marmesin ((+)-marmesin) shows COX-2/5-LOX dual inhibitory activity[2].
References:
[1]. Larbat R, et al. Molecular cloning and functional characterization of psoralen synthase, the first committed monooxygenase of furanocoumarin biosynthesis. J Biol Chem. 2007 Jan 5;282(1):542-54. Epub 2006 Oct 26.
[2]. Kim JS, et al. Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch Pharm Res. 2006 Aug;29(8):617-23.
- Ro 31-8220 Mesylate
Catalog No.:BCC4999
CAS No.:138489-18-6
- FK 888
Catalog No.:BCC5907
CAS No.:138449-07-7
- A 1070722
Catalog No.:BCC7933
CAS No.:1384424-80-9
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- Tinospinoside C
Catalog No.:BCN6925
CAS No.:1383977-51-2
- Afzelechin-(4alpha->8)-epiafzelechin
Catalog No.:BCN7709
CAS No.:1383627-30-2
- Boc-Arg(Tos)-OH
Catalog No.:BCC3067
CAS No.:13836-37-8
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- BD 1047 dihydrobromide
Catalog No.:BCC6863
CAS No.:138356-21-5
- BD 1008 dihydrobromide
Catalog No.:BCC6674
CAS No.:138356-09-9
- CYM 9484
Catalog No.:BCC6238
CAS No.:1383478-94-1
- Hedycoronen A
Catalog No.:BCN7653
CAS No.:1383441-73-3
- 3-Oxopomolic acid
Catalog No.:BCN3485
CAS No.:13849-90-6
- Pomolic acid
Catalog No.:BCN6197
CAS No.:13849-91-7
- Tormentic acid
Catalog No.:BCN6198
CAS No.:13850-16-3
- 9-Hydroxycanthin-6-one
Catalog No.:BCN3105
CAS No.:138544-91-9
- N3PT
Catalog No.:BCC1782
CAS No.:13860-66-7
- NKH 477
Catalog No.:BCC7126
CAS No.:138605-00-2
- (R)-3-Carboxy-4-hydroxyphenylglycine
Catalog No.:BCC6606
CAS No.:13861-03-5
- Securitinine
Catalog No.:BCN6986
CAS No.:13861-71-7
- LP 20 hydrochloride
Catalog No.:BCC6266
CAS No.:1386928-34-2
- Eugenol rutinoside
Catalog No.:BCN6201
CAS No.:138772-01-7
- Fmoc-D-1-Nal-OH
Catalog No.:BCC3284
CAS No.:138774-49-3
- Fmoc-D-2-Nal-OH
Catalog No.:BCC3290
CAS No.:138774-94-4
In vivo and molecular docking studies using whole extract and phytocompounds of Aegle marmelos fruit protective effects against Isoproterenol-induced Myocardial infarction in rats.[Pubmed:28511341]
Biomed Pharmacother. 2017 Jul;91:880-889.
Myocardial infarction (MI) is a leading major health problem with increased morbidity and mortality worldwide. The present study investigates isoproterenol (ISO) induced MI and the beneficial role of Aegle marmelos fruit extract (AMFE) in rats. Our results indicated the significant augmentation of plasma nitric oxide (NOx) levels, C-reactive protein (CRP), homocysteine, apolipoprotein B (apo-B), cardiac tissue lipid peroxidation and liver 3-hydroxy-3 methyl glutaryl CoA (HMG-CoA) reductase activity in ISO treated rats (85mg/kg b.wt) with a concomitant decrease in plasma apolipoprotein A1 (apo-A), lipase activity, paraoxonase-1 activity and cardiac tissue taurine levels when compared with controls. However, pretreatment of ISO administered rats with AMFE (150mg/kg b.wt/day for 45 days) markedly brought the observed alterations toward near normal level indicating its protective role against MI. Further, we have extended our studies to study the interaction of important phytocompounds, marmesin, marmin, umbelliferone and impertonin, present in AMFE with key enzymes, HMG-CoA reductase, iNOS, lipoprotein lipase and paraoxonase using AutoDock4. Molecular docking analysis indicated that HMG-CoA reductase, inducible nitric oxide synthase (iNOS) and lipoprotein lipase formed a strong enzyme ligand complex with impertonin. While the marmesin showed strong interaction with paraoxonase enzyme. In conclusion, our results suggest that AMFE acts as a strong protective agent against ISO-induced MI, and the bioactive compounds are responsible for this protective action which is confirmed by molecular docking studies.
Xylactam B, A New Isobenzofuranone from an Endophytic Xylaria sp.[Pubmed:26669110]
Nat Prod Commun. 2015 Oct;10(10):1715-7.
A new nitrogen containing compound named xylactam B (2), along with a further eight known compounds, ceramide 2a, cerebroside B, cyclo(prolyl,valyl), marmesin, 5-methoxycarbonylmellein, 5-methylmellein, polypropylene glycol and p-hydroxybenzoic acid, were isolated from an endophytic Xylaria sp. The structure elucidation of the new compound and the other isolates was carried out with the help of spectroscopic analyses and databases.
In vitro and in vivo anticancer effects of marmesin in U937 human leukemia cells are mediated via mitochondrial-mediated apoptosis, cell cycle arrest, and inhibition of cancer cell migration.[Pubmed:29251335]
Oncol Rep. 2018 Feb;39(2):597-602.
Leukemia is one of the highly lethal cancers among all pediatric cancers. With limited drug options and the severe side effects associated with the current chemotherapy, there is pressing need to look for new and novel anticancer agents. Against this backdrop, in the present study we evaluated the anticancer activity of a natural coumarin, marmesin against human leukemia cell line U937 and normal human monocytes It was observed that marmesin exhibited an IC50 value of 40 microM and exerted its cytotoxic effects in a dose-dependent manner. However, the cytotoxic effects of marmesin were comparatively lower for the normal human monocytes as evident from the IC50 of 125 microM. Our results indicated that marmesin inhibits colony formation and induces apoptosis dose-dependently. We also investigated the effect of marmesin on the expression of Bax and Bcl-2 proteins. It was observed that marmesin treatment triggered upregulation of Bax and downregulation of Bcl-2 causing significant increase in the Bax/Bcl-2 ratio, marmesin could also induce ROS mediated alterations in mitochondrial membrane potential. Additionally, marmesin induced G2/M cell cycle arrest and significantly inhibited cell migration potential of leukemia cells at the IC50. Remarkably, marmesin prevent tumor growth significantly in vivo at the dosage of 30 mg/kg in vivo. These results strongly indicate that marmesin may prove to be a novel anticancer lead for the management of leukemia.
Antioxidant, 5-lipoxygenase inhibitory and cytotoxic activities of compounds isolated from the Ferula lutea flowers.[Pubmed:25340301]
Molecules. 2014 Oct 22;19(10):16959-75.
A phytochemical investigation of the Ferula lutea (Poir.) Maire flowers has led to the isolation of a new compound, (E)-5-ethylidenefuran-2(5H)-one-5-O-beta-d-glucopyranoside (1), designated ferunide, 4-hydroxy-3-methylbut-2-enoic acid (2), reported for the first time as a natural product, together with nine known compounds, verbenone-5-O-beta-d-glucopyranoside (3), 5-O-caffeoylquinic acid (4), methyl caffeate (5), methyl 3,5-O-dicaffeoylquinate (6), 3,5-O-dicaffeoylquinic acid (7), isorhamnetin-3-O-alpha-l-rhamnopyranosyl(1-->6)-beta-d-glucopyranoside, narcissin (8), (-)-marmesin (9), isoimperatorin (10) and 2,3,6-trimethylbenzaldehyde (11). Compounds 3-10 were identified for the first time in Ferula genus. Their structures were elucidated by spectroscopic methods, including 1D and 2D NMR experiments, mass spectroscopy and X-ray diffraction analysis (compound 2), as well as by comparison with literature data. The antioxidant, anti-inflammatory and cytotoxic activities of isolated compounds were evaluated. Results showed that compound 7 exhibited the highest antioxidant activity with IC50 values of 18 +/- 0.5 micromol/L and 19.7 +/- 0.7 micromol/L by DPPH radical and ABTS radical cation, respectively. The compound 6 exhibited the highest anti-inflammatory activity with an IC50 value of 5.3 +/- 0.1 micromol/L against 5-lipoxygenase. In addition, compound 5 was found to be the most cytotoxic, with IC50 values of 22.5 +/- 2.4 micromol/L, 17.8 +/- 1.1 micromol/L and 25 +/- 1.1 micromol/L against the HCT-116, IGROV-1 and OVCAR-3 cell lines, respectively.
Marmesin is a novel angiogenesis inhibitor: Regulatory effect and molecular mechanism on endothelial cell fate and angiogenesis.[Pubmed:26455771]
Cancer Lett. 2015 Dec 28;369(2):323-30.
In the present study, we investigated the effects and molecular mechanism of marmesin, a coumarin compound isolated from Broussonetia kazinoki, on vascular endothelial growth factor-A (VEGF-A)-induced endothelial cell responses in vitro and angiogenic sprouting in aortic rings ex vivo. Marmesin treatment inhibited VEGF-A-stimulated endothelial cell proliferation through down-regulation of cell cycle-related proteins including cyclin-dependent kinases and cyclins, leading to pRb hypophosphorylation and G1 phase cell cycle arrest. In addition, marmesin treatment abrogated VEGF-A-induced endothelial cell migration, invasion and capillary-like structure formation in vitro as well as angiogenic sprouting ex vivo. These anti-angiogenic activities of marmesin were mediated through inactivation of VEGF-A-stimulated signaling pathways, and down-regulation of cell surface signaling molecules including VEGF receptor-2, human epidermal growth factor receptor-2, integrin beta1 and integrin-liked kinase. Taken together, these findings clearly support the pharmacological roles of marmesin in regulating angiogenesis, and warrant further evaluation and development as a potential therapeutic agent for the treatment and prevention of angiogenesis-related diseases including cancer.
[Chemical constituents from lipophilic parts in roots of Angelica dahurica var. formosana cv. Chuanbaizhi].[Pubmed:26552172]
Zhongguo Zhong Yao Za Zhi. 2015 Jun;40(11):2148-56.
The chemical constituents from lipophilic parts in the roots of Angelica dahurica var. formosana cv. Chuanbaizhi were studied in this paper. The compounds were separated and purified by repeated column chromatographic methods on silica gel and HPLC, and the chemical structures of compounds were determined by spectral data analyses. Twenty-nine compounds were obtained and identified as isoimperatorin (1), beta-sitosterol (2), imperatorin (3), bergapten (4), osthenol (5), xanthotoxin (6), isoimpinellin (7), dehydrogeijerin (8), phellopterin (9), isodemethylfuropinarine (10), 7-demethylsuberosin (11), alloimperatorin (12), xanthotoxol (13), isooxypeucedanin (14), alloisoimperatorin (15), demethylfuropinarine (16), 5-hydroxy-8-methoxypsoralen (17), oxypeucedanin methanolate (18), pabulenol (19), byakangelicin (20), marmesin (21), (+) -decursinol (22), heraclenol (23), oxypeucedanin hydrate (24), marmesinin (25), ulopterol (26), erythro-guaiacylglycerol-beta-ferulic acid ether (27), threo-guaiacylglycerol-beta-ferulic acid ether (28), and uracil (29). Compounds 5, 8, 11, 18, 21-23, and 26-28 were obtained from the roots of title plant for the first time.
Marmesin-mediated suppression of VEGF/VEGFR and integrin beta1 expression: Its implication in non-small cell lung cancer cell responses and tumor angiogenesis.[Pubmed:27878269]
Oncol Rep. 2017 Jan;37(1):91-97.
In the present study, we investigated the effects and molecular mechanism of marmesin, a natural coumarin compound isolated from Broussonetia kazinoki, on non-small cell lung cancer (NSCLC) cell responses and tumor angiogenesis. Marmesin abrogated mitogen-stimulated proliferation and invasion in both p53 wild-type A549 and p53-deficient H1299 NSCLC cells. These antitumor activities of marmesin were mediated by the inactivation of mitogenic signaling pathways and downregulation of cell signaling-related proteins including vascular endothelial growth factor receptor-2 (VEGFR-2), integrin beta1, integrin-linked kinase and matrix metalloproteinases-2. Furthermore, marmesin suppressed the expression and secretion of VEGF in both NSCLC cells, leading to inhibition of capillary-like structure formation in human umbilical vein endothelial cells. Collectively, these findings demonstrate the pharmacological roles and molecular targets of marmesin in regulating NSCLC cell responses and tumor angiogenesis.


