TranilastAngiogenesis inhibitor CAS# 53902-12-8 |
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- Irbesartan
Catalog No.:BCC2560
CAS No.:138402-11-6
- Olmesartan
Catalog No.:BCC1819
CAS No.:144689-24-7
- AVE 0991
Catalog No.:BCC4032
CAS No.:304462-19-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 53902-12-8 | SDF | Download SDF |
| PubChem ID | 5282230 | Appearance | Powder |
| Formula | C18H17NO5 | M.Wt | 327.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | MK 341; SB 252218 | ||
| Solubility | DMSO : 50 mg/mL (152.75 mM; Need ultrasonic) H2O : 10 mg/mL (30.55 mM; ultrasonic and adjust pH to 12 with NaOH) H2O : < 0.1 mg/mL (insoluble) | ||
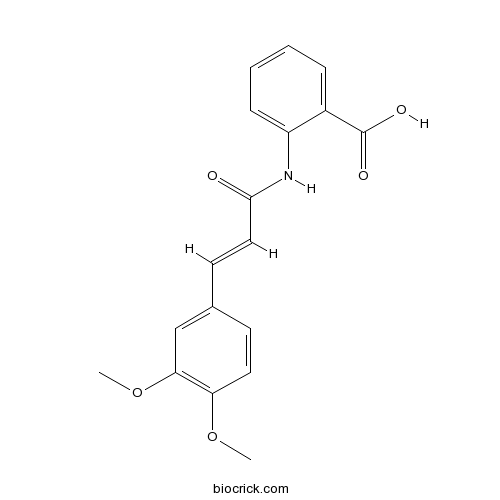
| Chemical Name | 2-[[(E)-3-(3,4-dimethoxyphenyl)prop-2-enoyl]amino]benzoic acid | ||
| SMILES | COC1=C(C=C(C=C1)C=CC(=O)NC2=CC=CC=C2C(=O)O)OC | ||
| Standard InChIKey | NZHGWWWHIYHZNX-CSKARUKUSA-N | ||
| Standard InChI | InChI=1S/C18H17NO5/c1-23-15-9-7-12(11-16(15)24-2)8-10-17(20)19-14-6-4-3-5-13(14)18(21)22/h3-11H,1-2H3,(H,19,20)(H,21,22)/b10-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antiallergic via inhibition of chemical mediator release from mast cells. Shown to be an effective inhibitor of angiogenesis. Demonstrated to antagonize the effects of angiotensin II on human arteries, possibly by an interaction at the level of the AT1 receptor. Inhibits TRPV2-mediated responses; binds to Aβ40 monomers and increases Aβ40 fibrillation. |

Tranilast Dilution Calculator

Tranilast Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.055 mL | 15.2751 mL | 30.5502 mL | 61.1004 mL | 76.3755 mL |
| 5 mM | 0.611 mL | 3.055 mL | 6.11 mL | 12.2201 mL | 15.2751 mL |
| 10 mM | 0.3055 mL | 1.5275 mL | 3.055 mL | 6.11 mL | 7.6376 mL |
| 50 mM | 0.0611 mL | 0.3055 mL | 0.611 mL | 1.222 mL | 1.5275 mL |
| 100 mM | 0.0306 mL | 0.1528 mL | 0.3055 mL | 0.611 mL | 0.7638 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Antiallergic via inhibition of chemical mediator release from mast cells. Most recently shown to be an effective inhibitor of angiogenesis. Demonstrated to antagonize the effects of angiotensin II on human arteries, possibly by an interaction at the level
- Allicin
Catalog No.:BCN2347
CAS No.:539-86-6
- Perillen
Catalog No.:BCN6527
CAS No.:539-52-6
- Hordenine
Catalog No.:BCN1424
CAS No.:539-15-1
- Ticlopidine HCl
Catalog No.:BCC4973
CAS No.:53885-35-1
- Desoxo-narchinol A
Catalog No.:BCN7636
CAS No.:53859-06-6
- Caboxine A
Catalog No.:BCN5715
CAS No.:53851-13-1
- 8-Prenylnaringenin
Catalog No.:BCN2998
CAS No.:53846-50-7
- Flavaprin
Catalog No.:BCN5714
CAS No.:53846-49-4
- 3-(beta-D-Glucopyranosyloxy)-2-hydroxybenzoic acid methyl ester
Catalog No.:BCN7734
CAS No.:53827-68-2
- Trifloroside
Catalog No.:BCN6915
CAS No.:53823-10-2
- Onitisin
Catalog No.:BCN5713
CAS No.:53823-03-3
- Onitin
Catalog No.:BCN5712
CAS No.:53823-02-2
- Nb-Feruloyltryptamine
Catalog No.:BCN3899
CAS No.:53905-13-8
- Pentostatin
Catalog No.:BCC1845
CAS No.:53910-25-1
- (+)-Anabasine hydrochloride
Catalog No.:BCC7219
CAS No.:53912-89-3
- Hederagenin 28-O-beta-D-glucopyranosyl ester
Catalog No.:BCN1423
CAS No.:53931-25-2
- Deoxyarbutin
Catalog No.:BCC4774
CAS No.:53936-56-4
- Crotalarine
Catalog No.:BCN2076
CAS No.:53937-97-6
- Euparone
Catalog No.:BCN7204
CAS No.:53947-86-7
- Apterin
Catalog No.:BCN3910
CAS No.:53947-89-0
- Corylin
Catalog No.:BCN5716
CAS No.:53947-92-5
- Aristolactam AII
Catalog No.:BCN3924
CAS No.:53948-07-5
- Aristolactam BII
Catalog No.:BCN5717
CAS No.:53948-09-7
- Aristolactam BIII
Catalog No.:BCN5718
CAS No.:53948-10-0
Evaluation of Suppressive Effects of Tranilast on the Invasion/Metastasis Mechanism in a Murine Pancreatic Cancer Cell Line.[Pubmed:28196028]
Pancreas. 2017 Apr;46(4):567-574.
OBJECTIVES: Numerous studies have investigated the mechanism of the antitumor effect of Tranilast, well known as an antiallergic drug. Herein, we investigated the mechanism of the antitumor effects of Tranilast using murine PAN 02 cell line. METHODS: In an allograft mouse model, the number of metastatic sites in the liver was counted. Wound healing and chemoinvasion assay were performed to evaluate migration and invasive ability of PAN 02, respectively. Activities of matrix metalloproteinases (MMPs) were evaluated by gelatin zymography. The expression of cofactors in the activation of MMP-2 was assessed by immunohistochemical staining at the front of metastasis. RESULTS: The number of metastatic sites was reduced in Tranilast-treated groups. Migration ability and tumor invasiveness were significantly inhibited by Tranilast in a dose-dependent manner. Gelatin zymography revealed inhibition of MMP-2 activity. Immunohistochemical staining showed remarkable attenuation of tissue inhibitor of metalloproteinase (TIMP-) 2 expression in Tranilast-treated groups. CONCLUSIONS: Tissue inhibitor of metalloproteinase 2 is necessary for MMP-2 activation with interaction between membrane type 1-MMP and proMMP-2. These results suggested that Tranilast may inhibit MMP-2 activation through attenuating TIMP-2 expression, resulting in inhibition of tumor invasion and metastasis. Our results showed possibility of Tranilast in clinical application for novel cancer therapy.
Uricosuric targets of tranilast.[Pubmed:28357121]
Pharmacol Res Perspect. 2017 Feb 6;5(2):e00291.
Uric acid, generated from the metabolism of purines, has both proven and emerging roles in human disease. Serum uric acid in humans is determined by production and by the net balance of reabsorption and secretion in kidney and intestine. In the human kidney, epithelial reabsorption dominates over secretion, such that in normal subjects there is at least 90% net reabsorption of filtered urate resulting in a fractional excretion of <10%. Tranilast, an anti-inflammatory drug with pleiotropic effects, has a marked hypouricemic, uricosuric effect in humans. We report here that Tranilast is a potent inhibitor of [(14)C]-urate transport mediated by the major reabsorptive urate transporters (URAT1, GLUT9, OAT4, and OAT10) in Xenopus oocytes; this provides an unequivocal molecular mechanism for the drug's uricosuric effect. Tranilast was found to inhibit urate transport mediated by URAT1 and GLUT9 in a fully reversible and noncompetitive (mixed) manner. In addition, Tranilast inhibits the secretory urate transporters NPT1, OAT1, and OAT3 without affecting the secretory efflux pump ABCG2. Notably, while benzbromarone and probenecid inhibited urate as well as nicotinate transport, Tranilast inhibited the urate transport function of URAT1, GLUT9, OAT4, OAT10, and NPT1, without significantly affecting nicotinate transport mediated by SMCT1 (IC 50 ~1.1 mmol/L), SMCT2 (IC 50 ~1.0 mmol/L), and URAT1 (IC 50 ~178 mumol/L). In summary, Tranilast causes uricosuria by inhibiting all the major reabsorptive urate transporters, selectively affecting urate over nicotinate transport. These data have implications for the treatment of hyperuricemia and gout, the pharmacology of Tranilast, and the structure-function analysis of urate transport.
Tranilast Inhibits Genes Functionally Involved in Cell Proliferation, Fibrosis, and Epigenetic Regulation and Epigenetically Induces miR-29c Expression in Leiomyoma Cells.[Pubmed:28114878]
Reprod Sci. 2017 Sep;24(9):1253-1263.
Tranilast (N-3,4-dimethoxycinnamoyl anthranilic acid) is an antiallergic agent with inhibitory effects on cell proliferation and extracellular matrix production. Here we assess the effect of Tranilast on the expression of miR-29c and genes functionally involved in cell proliferation, fibrosis, and epigenetic regulation in isolated leiomyoma smooth muscle cells (LSMC). Tranilast significantly inhibited the rate of LSMC proliferation, which was associated with downregulation of cell cycle progression genes cyclin D1 (CCND1) and cyclin-dependent kinase 2 (CDK2) expression at messenger RNA and protein levels ( P < .05). Tranilast also suppressed the expression of collagen type I (COL1), collagen type III alpha 1 chain (COL3A1), the profibrotic cytokine, transforming growth factor beta-3 (TGF-beta3), DNA (cytosine-5)-methyltransferase 1 (DNMT1), and enhancer of zeste homolog 2 (EZH2), which regulate epigenetic status of gene promoters ( P < .05). Tranilast also significantly induced the expression of cellular and secreted miR-29c through downregulation of methylation status of miR-29c promoter ( P < .05). In addition, Tranilast suppressed the activity of luciferase reporter containing 3'UTR of COL3A1 and CDK2, which are downstream targets of miR-29c ( P < .05). Knockdown of miR-29c expression attenuated the inhibitory effects of Tranilast on COL3A1 and CDK2 protein expression ( P < .05). Collectively, these findings suggest that Tranilast could have therapeutic potential as an inhibitory agent for leiomyoma growth and its associated symptoms.
Increased dissolution rates of tranilast solid dispersions extruded with inorganic excipients.[Pubmed:28122459]
Drug Dev Ind Pharm. 2017 Jun;43(6):947-957.
The purpose of this study was to evaluate the performance of Neusilin(R) (NEU) a synthetic magnesium aluminometasilicate as an inorganic drug carrier co-processed with the hydrophilic surfactants Labrasol and Labrafil to develop Tranilast (TLT)-based solid dispersions using continuous melt extrusion (HME) processing. Twin-screw extrusion was optimized to develop various TLT/excipient/surfactant formulations followed by continuous capsule filling in the absence of any downstream equipment. Physicochemical characterization showed the existence of TLT in partially crystalline state in the porous network of inorganic NEU for all extruded formulations. Furthermore, in-line NIR studies revealed a possible intermolecular H-bonding formation between the drug and the carrier resulting in the increase of TLT dissolution rates. The capsules containing TLT-extruded solid dispersions showed enhanced dissolution rates and compared with the marketed Rizaben((R)) product.
Tranilast binds to abeta monomers and promotes abeta fibrillation.[Pubmed:23679559]
Biochemistry. 2013 Jun 11;52(23):3995-4002.
The antiallergy and potential anticancer drug Tranilast has been patented for treating Alzheimer's disease (AD), in which amyloid beta-protein (Abeta) plays a key pathogenic role. We used solution NMR to determine that Tranilast binds to Abeta40 monomers with approximately 300 muM affinity. Remarkably, Tranilast increases Abeta40 fibrillation more than 20-fold in the thioflavin T assay at a 1:1 molar ratio, as well as significantly reducing the lag time. Tranilast likely promotes fibrillation by shifting Abeta monomer conformations to those capable of seed formation and fibril elongation. Molecular docking results qualitatively agree with NMR chemical shift perturbation, which together indicate that hydrophobic interactions are the major driving force of the Abeta-Tranilast interaction. These data suggest that AD may be a potential complication for Tranilast usage in elderly patients.
Regulation of calcium-permeable TRPV2 channel by insulin in pancreatic beta-cells.[Pubmed:18984736]
Diabetes. 2009 Jan;58(1):174-84.
OBJECTIVE: Calcium-permeable cation channel TRPV2 is expressed in pancreatic beta-cells. We investigated regulation and function of TRPV2 in beta-cells. RESEARCH DESIGN AND METHODS: Translocation of TRPV2 was assessed in MIN6 cells and cultured mouse beta-cells by transfecting TRPV2 fused to green fluorescent protein or TRPV2 containing c-Myc tag in the extracellular domain. Calcium entry was assessed by monitoring fura-2 fluorescence. RESULTS: In MIN6 cells, TRPV2 was observed mainly in cytoplasm in an unstimulated condition. Addition of exogenous insulin induced translocation and insertion of TRPV2 to the plasma membrane. Consistent with these observations, insulin increased calcium entry, which was inhibited by Tranilast, an inhibitor of TRPV2, or by knockdown of TRPV2 using shRNA. A high concentration of glucose also induced translocation of TRPV2, which was blocked by nefedipine, diazoxide, and somatostatin, agents blocking glucose-induced insulin secretion. Knockdown of the insulin receptor attenuated insulin-induced translocation of TRPV2. Similarly, the effect of insulin on TRPV2 translocation was not observed in a beta-cell line derived from islets obtained from a beta-cell-specific insulin receptor knockout mouse. Knockdown of TRPV2 or addition of Tranilast significantly inhibited insulin secretion induced by a high concentration of glucose. Likewise, cell growth induced by serum and glucose was inhibited by Tranilast or by knockdown of TRPV2. Finally, insulin-induced translocation of TRPV2 was observed in cultured mouse beta-cells, and knockdown of TRPV2 reduced insulin secretion induced by glucose. CONCLUSIONS: TRPV2 is regulated by insulin and is involved in the autocrine action of this hormone on beta-cells.
Tranilast, an anti-allergic drug, possesses antagonistic potency to angiotensin II.[Pubmed:9865509]
Eur J Pharmacol. 1998 Nov 20;361(2-3):199-205.
N-(3',4'-dimethoxycinnamoyl) anthranilic acid (Tranilast), an effective anti-allergic drug, has successfully prevented restenosis in patients who have undergone percutaneous transluminal coronary angioplasty. To elucidate the mechanism of Tranilast, we investigated its antagonistic effect to angiotensin II, which plays a pivotal role in the proliferation of vascular smooth muscle cells, using angiotensin II-induced contractions in human gastroepiploic artery and rabbit aorta. The possible antagonistic effects of other anti-allergic agents such as 4-( p-chlorobenzyl)-2-(hexahydro-1-methyl-1H-azepin-4-yl)-1(2H)-phthal azinone hydrochloride (azelastine), 9-methyl-3-( 1H-tetrazol-5-yl)-4H-pyrido[1,2-a]pyramidin-4-one potassium salt (pemirolast) and disodium cromoglycate were also compared. Tranilast dose-dependently inhibited the angiotensin II-induced contractions in human and rabbit arteries (IC50 = 3.6x10(-5) M and pD'2 = 3.69, respectively). Pemirolast showed a weak antagonistic effect to angiotensin II, but the effective concentration cannot be administered in clinical dosage. Tranilast and pemirolast had no effect on the concentration-contractile response curves for KCI and norepinephrine. Azelastine inhibited angiotensin II-, KCl- and norepinephrine-induced contractions non-specifically, while disodium cromoglycate did not affect these contractile responses. Tranilast but not azelastine showed synergistic action with 2-ethoxy-1-[[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl]-1H-benzimi dazole-7-carboxylic acid (CV- 11974) in antagonizing angiotensin II-induced contraction and the inhibitory pattern was similar to that of the non-peptide angiotensin II AT1 receptor antagonist CV-11974. These findings indicate that only Tranilast possesses the unique ability to antagonize angiotensin II in clinical dosage, which may contribute at least in part to prevention of restenosis after percutaneous transluminal coronary angioplasty.
Tranilast inhibits the proliferation, chemotaxis and tube formation of human microvascular endothelial cells in vitro and angiogenesis in vivo.[Pubmed:9401770]
Br J Pharmacol. 1997 Nov;122(6):1061-6.
1. First developed as an antiallergic drug, Tranilast inhibits chemical mediator release from mast cells. In the present study, we examine the effects of Tranilast on angiogenesis in vitro and in vivo and discuss the application of Tranilast for angiogenic diseases. 2. Tranilast inhibited significantly the proliferation (IC50: 136 microM, 95% confidence limits: 134-137 microM) and vascular endothelium growth factor (VEGF)-induced chemotaxis (IC50: 135 microM, 95% confidence limits: 124-147 microM) of human dermal microvascular endothelial cells (HDMECs) at concentrations greater than 25 micrograms ml-1. No toxicity to HDMECs measuring by LDH release and no inhibitory effects on metalloproteinase (MMP)-2 and MMP-9 activity were observed even at 100 micrograms ml-1 (306 microM). 3. Tube formation of HDMECs cultured on the matrigel as an in vitro angiogenesis model was inhibited by Tranilast in a concentration-dependent manner. The IC50 value and 95% confidence limits were 175 microM and 151-204 microM, respectively. 4. In vivo angiogenesis was induced in mice by the subcutaneous injection of matrigel containing 30 ng ml-1 VEGF and 64 micrograms ml-1 heparin. Tranilast was administered orally twice a day for 3 days. Tranilast dose-dependently suppressed angiogenesis in the matrigel and a significant change was observed at a dose of 300 mg kg-1. 5. These results indicate that Tranilast is an angiogenesis inhibitor which may be beneficial for the improvement of angiogenic diseases such as proliferative diabetic retinopathy, age-related macular degeneration, tumour invasion and rheumatoid arthritis.
Tranilast restores cytokine-induced nitric oxide production against platelet-derived growth factor in vascular smooth muscle cells.[Pubmed:8856474]
J Cardiovasc Pharmacol. 1996 Aug;28(2):200-7.
Tranilast has been reported to reduce restenosis rate after angioplasty, but its mechanism is still unclear. We investigated the effect of Tranilast against platelet-derived growth factor (PDGF) in PDGF's proliferative effect and PDGF's inhibitory effect on cytokine-induced nitric oxide (NO) production in vascular smooth muscle cells (VSMC). NO production was measured by Griess reaction. NO synthase (NOS) protein was evaluated by Western blot with monoclonal anti-rat inducible NOS antibody. A combination of interleukin-1 beta (IL-1 beta 1 ng/ml), tumor necrosis factor-alpha (TNF-alpha 2,000 U/ml), and lipopolysaccharide (100 ng/ml) significantly increased NO production and NOS protein, and Tranilast significantly enhanced both in a dose-dependent manner. PDGF (100 ng/ml) significantly reduced both cytokine-induced NO production and NOS protein induction, but Tranilast completely abolished these inhibitory effects. In the presence of cytokines, serum-stimulated cell proliferation was significantly inhibited by cytokine-induced NO, whereas PDGF-stimulated proliferation was not. On the other hand, Tranilast not only inhibited the proliferative effect of PDGF directly, but also restored cytokine-induced NO production and its antiproliferative effect in the presence of PDGF.


