CarpachromeneCAS# 57498-96-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57498-96-1 | SDF | Download SDF |
| PubChem ID | 10449654 | Appearance | Yellow powder |
| Formula | C20H16O5 | M.Wt | 336.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
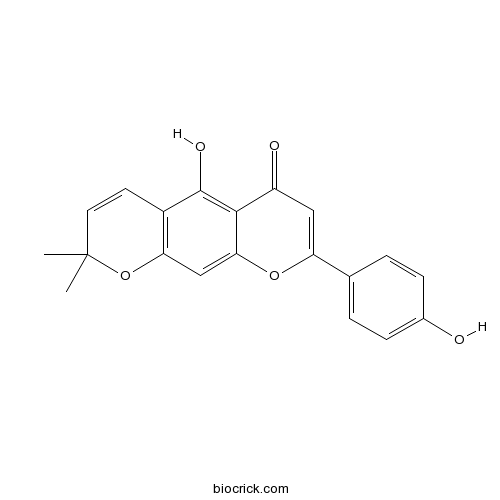
| Chemical Name | 5-hydroxy-8-(4-hydroxyphenyl)-2,2-dimethylpyrano[3,2-g]chromen-6-one | ||
| SMILES | CC1(C=CC2=C(O1)C=C3C(=C2O)C(=O)C=C(O3)C4=CC=C(C=C4)O)C | ||
| Standard InChIKey | YXOATFKTEDZPFL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H16O5/c1-20(2)8-7-13-16(25-20)10-17-18(19(13)23)14(22)9-15(24-17)11-3-5-12(21)6-4-11/h3-10,21,23H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Carpachromene blocks protein expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in LPS-stimulated macrophages, it could be a potential anti-inflammatory agent. 2. Carpachromene exhibits significant cytotoxicity against HepG2, PLC/PRF/5 and Raji cancer cell lines in vitro. 3. Carpachromene shows significant α-glucosidase inhibitory activity. |
| Targets | NO | TNF-α | NOS | COX |

Carpachromene Dilution Calculator

Carpachromene Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9735 mL | 14.8677 mL | 29.7354 mL | 59.4707 mL | 74.3384 mL |
| 5 mM | 0.5947 mL | 2.9735 mL | 5.9471 mL | 11.8941 mL | 14.8677 mL |
| 10 mM | 0.2974 mL | 1.4868 mL | 2.9735 mL | 5.9471 mL | 7.4338 mL |
| 50 mM | 0.0595 mL | 0.2974 mL | 0.5947 mL | 1.1894 mL | 1.4868 mL |
| 100 mM | 0.0297 mL | 0.1487 mL | 0.2974 mL | 0.5947 mL | 0.7434 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Angeflorin
Catalog No.:BCN6656
CAS No.:57498-69-8
- Carbasalate calcium
Catalog No.:BCC8904
CAS No.:5749-67-7
- BRL-54443
Catalog No.:BCC5047
CAS No.:57477-39-1
- 16-Deoxysaikogenin F
Catalog No.:BCN6414
CAS No.:57475-62-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
- H-Asn-OMe.HCl
Catalog No.:BCC2876
CAS No.:57461-34-4
- Stigmasta-3,5-diene
Catalog No.:BCC8254
CAS No.:4970-37-0
- 2-Epi-3a-epiburchellin
Catalog No.:BCN7013
CAS No.:57457-99-5
- p-Menth-8-ene-1,2-diol
Catalog No.:BCN5777
CAS No.:57457-97-3
- Resiniferatoxin
Catalog No.:BCC6951
CAS No.:57444-62-9
- Methylergometrine maleate
Catalog No.:BCC6691
CAS No.:57432-61-8
- (2S)-2alpha-(1,3-Benzodioxol-5-yl)-3,5-dihydro-5alpha-methoxy-3beta-methyl-5-allyl-2H-benzofuran-6-one
Catalog No.:BCN6606
CAS No.:57430-03-2
- 3,4'-Di-O-methylellagic acid
Catalog No.:BCN3710
CAS No.:57499-59-9
- 3-Benzalphthalide
Catalog No.:BCC8621
CAS No.:575-61-1
- 6-Benzyloxypurine
Catalog No.:BCC8770
CAS No.:57500-07-9
- 2-Methylsulfanylpyrimidin-4(3H)-one
Catalog No.:BCC8582
CAS No.:5751-20-2
- H-His(Nτ-Me)-OMe.2HCl
Catalog No.:BCC2958
CAS No.:57519-09-2
- Notoginsenoside S
Catalog No.:BCN8371
CAS No.:575446-95-6
- SNOG
Catalog No.:BCC6714
CAS No.:57564-91-7
- Isofuranodiene
Catalog No.:BCN5781
CAS No.:57566-47-9
- PNU 37883 hydrochloride
Catalog No.:BCC7262
CAS No.:57568-80-6
- (3beta,24xi)-Cycloartane-3,24,25-triol
Catalog No.:BCN5782
CAS No.:57576-29-1
- (24S)-Cycloartane-3,24,25-triol 24,25-acetonide
Catalog No.:BCN1414
CAS No.:57576-31-5
- (2,4-Dihydroxyphenyl)acetonitrile
Catalog No.:BCN5783
CAS No.:57576-34-8
Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent.[Pubmed:18683821]
Mol Nutr Food Res. 2008 Dec;52(12):1530-8.
A new furanoflavone, 7-(2,4-dihydroxyphenyl)-4-hydroxy-2-(2-hydroxy propan-2-yl)-2, 3-dihydrofuro(3, 2-g)chromen-5-one (artocarpfuranol, 1), together with 14 known compounds, dihydromorin (2), steppogenin (3), norartocarpetin (4), artocarpanone (5), artocarpesin (6), artocarpin (7), cycloartocarpin (8), cycloartocarpesin (9), artocarpetin (10), brosimone I (11), cudraflavone B (12), Carpachromene (13), isoartocarpesin (14), and cyanomaclurin (15) were isolated from the wood of Artocarpus heterophyllus. Their structures were identified by interpretation of MS,( 1)H-NMR,( 13)C-NMR, HMQC, and HMBC spectroscopic data. Among them, compounds 1-6 and 14 showed strong mushroom tyrosinase inhibitory activity with IC(50) values lower than 50 microM, more potent than kojic acid (IC(50) = 71.6 microM), a well-known tyrosinase inhibitor. In addition, extract of A. heterophyllus was evaluated for its antibrowning effect on fresh-cut apple slices. It was discovered that fresh-cut apple slices treated by dipping in solution of 0.03 or 0.05% of A. heterophyllus extract with 0.5% ascorbic acid did not undergo any substantial browning reaction after storage at room temperature for 24 h. The antibrowning effect was significantly better than samples treated with the extract (0.03 or 0.05%) or ascorbic acid (0.5%) alone. The results provide preliminary evidence supporting the potential of this natural extract as antibrowning agent in food systems.
alpha-Glucosidase and 15-Lipoxygenase Inhibitory Activities of Phytochemicals from Calophyllum symingtonianum.[Pubmed:26594765]
Nat Prod Commun. 2015 Sep;10(9):1585-7.
A phytochemical investigation of the crude extracts of the bark and leaves of Calophyllum symingtonianum has resulted in the isolation of inophyllum D, inophyllum H, calanone, isocordato-oblongic acid, amentoflavone, Carpachromene and lupenone. Their chemical structures were elucidated and confirmed by spectroscopic analysis. All flavonoids and coumarins showed significant alpha-glucosidase inhibitory activity, while amentoflavone gave a positive result against 15-lipoxygenase inhibition.
Cytotoxic flavonoids and new chromenes from Ficus formosana f. formosana.[Pubmed:16395655]
Planta Med. 2005 Dec;71(12):1165-7.
Two new chromenes, ficuformodiol A and ficuformodiol B, and nine known compounds including one chromene, spatheliachromen, six flavonoids, obovatin, Carpachromene ( 5), apigenin ( 6), norartocarpetin ( 7), steppogenin, 6-prenylpinocembrin, one benzopyran, chromenylacrylic acid, and one isocoumarin, ( R)-(-)-mellein, were obtained from the cytotoxic chloroform-soluble fraction of the stems of Ficus formosana f. formosana (Moraceae). Their structures were determined by means of spectroscopic analyses. Compounds 5, 6 and 7 exhibited significant cytotoxicity against HepG2, PLC/PRF/5 and Raji cancer cell lines in vitro.
Bioassay guided isolation and identification of anti-inflammatory active compounds from the root of Ficus formosana.[Pubmed:24200240]
J Agric Food Chem. 2013 Nov 20;61(46):11008-15.
Activity-directed fractionation and purification processes were employed to identify the anti-inflammatory active compounds using lipopolysaccharide (LPS)-stimulated mouse macrophage (RAW264.7) in vitro. Air-dried roots of Ficus formosana were extracted with methanol and separated into n-hexane, chloroform, ethyl acetate, n-butanol, and water layers. Among them, the chloroform layer showed strong activity and was subjected to separation and purification by using various chromatographic techniques. Five compounds showing potent activity were identified by comparing spectral data to be beta-sitosterol, stigmasterol, psoralen, kaempferol, Carpachromene, and syringic aldehyde. When macrophages were treated with psoralen and kaempferol together with LPS, a concentration-dependent inhibition of nitric oxide (NO) and tumor necrosis factor (TNF-alpha) productions were detected. Western blotting revealed that kaempferol, psoralen, and Carpachromene blocked protein expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in LPS-stimulated macrophages. The results confirmed that the traditional use of F. formosana could be a potential anti-inflammatory agent.


