Carbasalate calciumCAS# 5749-67-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5749-67-7 | SDF | Download SDF |
| PubChem ID | 21975 | Appearance | Powder |
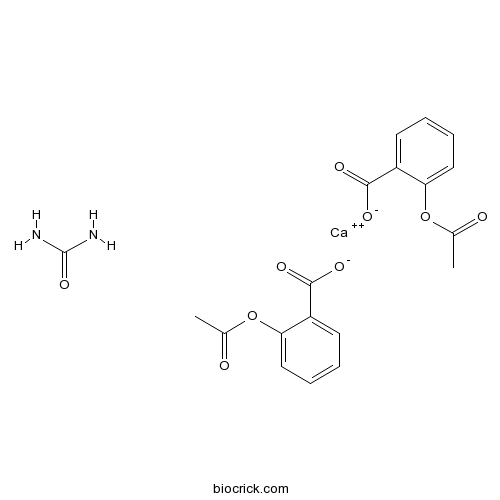
| Formula | C19H18CaN2O9 | M.Wt | 458.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 103 mg/mL (397.32 mM; Need ultrasonic and warming) | ||
| Chemical Name | calcium;2-acetyloxybenzoate;urea | ||
| SMILES | CC(=O)OC1=CC=CC=C1C(=O)[O-].CC(=O)OC1=CC=CC=C1C(=O)[O-].C(=O)(N)N.[Ca+2] | ||
| Standard InChIKey | VYMUGTALCSPLDM-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/2C9H8O4.CH4N2O.Ca/c2*1-6(10)13-8-5-3-2-4-7(8)9(11)12;2-1(3)4;/h2*2-5H,1H3,(H,11,12);(H4,2,3,4);/q;;;+2/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Carbasalate calcium Dilution Calculator

Carbasalate calcium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1815 mL | 10.9075 mL | 21.815 mL | 43.63 mL | 54.5375 mL |
| 5 mM | 0.4363 mL | 2.1815 mL | 4.363 mL | 8.726 mL | 10.9075 mL |
| 10 mM | 0.2182 mL | 1.0908 mL | 2.1815 mL | 4.363 mL | 5.4538 mL |
| 50 mM | 0.0436 mL | 0.2182 mL | 0.4363 mL | 0.8726 mL | 1.0908 mL |
| 100 mM | 0.0218 mL | 0.1091 mL | 0.2182 mL | 0.4363 mL | 0.5454 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BRL-54443
Catalog No.:BCC5047
CAS No.:57477-39-1
- 16-Deoxysaikogenin F
Catalog No.:BCN6414
CAS No.:57475-62-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
- H-Asn-OMe.HCl
Catalog No.:BCC2876
CAS No.:57461-34-4
- Stigmasta-3,5-diene
Catalog No.:BCC8254
CAS No.:4970-37-0
- 2-Epi-3a-epiburchellin
Catalog No.:BCN7013
CAS No.:57457-99-5
- p-Menth-8-ene-1,2-diol
Catalog No.:BCN5777
CAS No.:57457-97-3
- Resiniferatoxin
Catalog No.:BCC6951
CAS No.:57444-62-9
- Methylergometrine maleate
Catalog No.:BCC6691
CAS No.:57432-61-8
- (2S)-2alpha-(1,3-Benzodioxol-5-yl)-3,5-dihydro-5alpha-methoxy-3beta-methyl-5-allyl-2H-benzofuran-6-one
Catalog No.:BCN6606
CAS No.:57430-03-2
- 8-O-Acetylshanzhiside methyl ester
Catalog No.:BCN5776
CAS No.:57420-46-9
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
- Angeflorin
Catalog No.:BCN6656
CAS No.:57498-69-8
- Carpachromene
Catalog No.:BCN5779
CAS No.:57498-96-1
- 3,4'-Di-O-methylellagic acid
Catalog No.:BCN3710
CAS No.:57499-59-9
- 3-Benzalphthalide
Catalog No.:BCC8621
CAS No.:575-61-1
- 6-Benzyloxypurine
Catalog No.:BCC8770
CAS No.:57500-07-9
- 2-Methylsulfanylpyrimidin-4(3H)-one
Catalog No.:BCC8582
CAS No.:5751-20-2
- H-His(Nτ-Me)-OMe.2HCl
Catalog No.:BCC2958
CAS No.:57519-09-2
- Notoginsenoside S
Catalog No.:BCN8371
CAS No.:575446-95-6
- SNOG
Catalog No.:BCC6714
CAS No.:57564-91-7
- Isofuranodiene
Catalog No.:BCN5781
CAS No.:57566-47-9
- PNU 37883 hydrochloride
Catalog No.:BCC7262
CAS No.:57568-80-6
- (3beta,24xi)-Cycloartane-3,24,25-triol
Catalog No.:BCN5782
CAS No.:57576-29-1
Quantitative determination of carbasalate calcium derived metabolites, acetylsalicylic acid and salicylic acid, in six animal foods using liquid-liquid extraction method coupled with liquid chromatography-tandem mass spectrometry.[Pubmed:30583437]
Food Chem. 2019 Apr 25;278:744-750.
This work describes a simple screening protocol for quantification of Carbasalate calcium derived metabolites, acetylsalicylic acid (ASA) and salicylic acid (SA), in animal and aquatic food matrices using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The analytes were extracted from porcine muscle, milk, egg, shrimp, eel, and flatfish using acetonitrile, with the addition of formic acid as well as trifluoroacetic acid, followed by liquid-liquid purification with saturated n-hexane. A reverse-phase analytical column was employed with a mobile phase comprising (A) 1mM ammonium acetate in distilled water and (B) methanol to achieve the best chromatographic separation. Matrix-matched calibration curves (R(2)>/=0.9817) were constructed using six concentrations of 5, 10, 20, 30, 40, and 50microg/kg in porcine muscle, milk, egg, shrimp, eel, and flatfish matrices. The calculated limits of quantification (LOQ) were 10 and 7microg/kg, for ASA and SA, respectively. Recoveries of 67 to 102% with relative standard deviations (RSDs) of
Effect of anti-inflammatory drugs on colibacillosis lesions in broilers after Infectious Bronchitis Virus and subsequent Escherichia coli infection.[Pubmed:22475186]
Vet Q. 2012;32(1):25-9.
BACKGROUND: In case of persistent and sterile inflammation, anti-inflammatory drugs should be considered as first choice treatment instead of antibiotics. OBJECTIVE: To assess the effect of anti-inflammatory drugs on lesions due to colibacillosis. ANIMALS & METHODS: Five groups of day-old broilers of 15 birds each were housed in isolators and were inoculated at 29 days of age with Infectious Bronchitis Virus strain M41 by the oculo-nasal and IT (intratracheal) route (10(5.4) EID(50) (egg infectious dosis 50)/broiler) and at 33 days of age with Escherichia coli strain 506 by the IT route (10(7.6) colony forming units/broiler). Broilers of four groups were treated from day 28 up to and including day 39 orally on a daily basis with either Carbasalate calcium (4 x 12.5 mg), meloxicam (2 x 0.5 mg), acetaminophen (4 x 2.5 mg), or dexamethasone (1 x 1.0 mg). The fifth group was placebo-medicated. At 40 days of age, the experiment was ended and at post-mortem examination, colibacillosis lesions were assessed. RESULTS: All broilers in the dexamethasone group died. This mortality exceeded significantly (p < 0.05) that of the other groups in which mortality ranged from 2 to 5. Mean lesion score of surviving broilers of medicated groups ranged from 5.3 to 5.8 compared to 3.9 in the placebo group and did not differ significantly between groups. CONCLUSION: None of the anti-inflammatory drugs had a positive effect on colibacillosis lesions. CLINICAL IMPORTANCE: Anti-inflammatory drugs cannot be considered as an alternative for antibiotic treatment.
Analytical determination and pharmacokinetics of major metabolites of carbasalate calcium in broilers following oral administration.[Pubmed:21091728]
J Vet Pharmacol Ther. 2011 Aug;34(4):410-6.
As a newer anti-inflammatory agent, Carbasalate calcium is used in various animal species. In this study, the pharmacokinetics of Carbasalate calcium was investigated in broilers. Broilers, with body weight of 2.0 +/- 0.3 kg, were administrated Carbasalate calcium soluble powder at a single dose of 40 mg/kg body weight orally. The plasma concentrations of its metabolites, aspirin (ASA), salicylic acid (SA) and gentisic acid (GA) were determined by LC-MS/MS method and the pharmacokinetic parameters were calculated by noncompartmental analysis. After oral administration of Carbasalate calcium, the plasma drug concentration for ASA, SA and GA reached a peak (C(max) ) of 8.88 +/- 1.31, 42.6 +/- 4.62 and 10.1 +/- 2.16 mug/mL at 0.170, 2.00 and 2.00 h, respectively. The terminal half-life (t(1/2lambdaz) ) of ASA, SA and GA was 11.2 +/- 8.04, 23.7 +/- 17.1 and 28.6 +/- 4.90 h, respectively. In conclusion, analytical method for the quantification of ASA, SA and GA in plasma in the broilers was developed and validated. In broilers, Carbasalate calcium is quickly metabolized in ASA and ASA is rapidly converted to SA and one of the metabolites of SA is GA.
Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study.[Pubmed:19364926]
Arch Neurol. 2009 Jun;66(6):714-20.
BACKGROUND: Cerebral microbleeds are hemosiderin deposits in the brain that are indicative of microangiopathy. Microbleeds in strictly lobar brain locations have been related to cerebral amyloid angiopathy, a bleeding-prone disease state. OBJECTIVE: To investigate the relation between antithrombotic drug use and the presence of cerebral microbleeds, especially those in strictly lobar locations. DESIGN: A population-based, cross-sectional analysis that used magnetic resonance imaging (MRI) to assess the presence and location of microbleeds. Complete information on outpatient use of platelet aggregation inhibitors and anticoagulant drugs before MRI was obtained from automated pharmacy records. SETTING: The Rotterdam Scan Study, a population-based imaging study in a general elderly community in the Netherlands. PARTICIPANTS: A population-based sample of 1062 persons from a longitudinal cohort, 60 years and older, free of dementia, who underwent MRI examinations between August 15, 2005, and November 22, 2006. MAIN OUTCOME MEASURES: Presence of cerebral microbleeds on MRI. RESULTS: Compared with nonusers of antithrombotic drugs, cerebral microbleeds were more prevalent among users of platelet aggregation inhibitors (adjusted odds ratio [OR], 1.71; 95% confidence interval [CI], 1.21-2.41). We did not find a significant association for anticoagulant drugs and microbleed presence (OR, 1.49; 95% CI, 0.82-2.71). Strictly lobar microbleeds were more prevalent among aspirin users (adjusted OR compared with nonusers, 2.70; 95% CI, 1.45-5.04) than among persons using Carbasalate calcium (adjusted OR, 1.16; 95% CI, 0.66-2.02). This difference was even more pronounced when comparing persons who had used similar dosages of both drugs. CONCLUSIONS: This cross-sectional study shows that use of platelet aggregation inhibitors is related to the presence of cerebral microbleeds. Furthermore, aspirin and Carbasalate calcium use may differently relate to the presence of strictly lobar microbleeds.
A retrospective analysis of patients treated for superficial vein thrombosis.[Pubmed:19011268]
Neth J Med. 2008 Nov;66(10):423-7.
INTRODUCTION: The absolute risk of deep venous thrombosis (DVT) and pulmonary embolism (PE) as well as extension and/or recurrence in superficial vein thrombosis (SVT) of the leg is considerable and underestimated. We retrospectively evaluated therapeutic management, thrombophilic risk factors and clinical outcome of SVT. METHODS: A database search was performed for consecutive patients with a suspected SVT of the lower extremities referred to our institution between 1 January 1999 and 31 December 2004. The primary outcome measure was pain reduction at follow-up. Secondary outcome measures were progression or recurrence of SVT in the leg and the occurrence of (a)symptomatic DVT or symptomatic PE at follow-up. RESULTS: In 73 patients follow-up information was present (3/76 non-evaluable patients). In 9/32 (28%) of the patients treated with Carbasalate calcium, there was progression of SVT as assessed by ultrasonographic evaluation, compared with 3/11 (27%) in the low-molecular-weight heparin (LMWH) group and 3/6 (50%) in the no treatment group. DVT was diagnosed in 5/36 (14%) of the patients treated with Carbasalate calcium compared with 1/13 (1%) in the LMWH and 1/3 (33%) in the other treatment groups at follow-up. Furthermore, 34 were tested for thrombophilic defects, 27 of whom had one or more thrombophilic defect. CONCLUSION: The results of our study show that SVT may be prone to venous thromboembolism and therefore needs to be treated or carefully followed up.
Recurrent perimesencephalic subarachnoid hemorrhage during antithrombotic therapy.[Pubmed:18972074]
Neurocrit Care. 2009;10(2):209-12.
INTRODUCTION: In patients with non-aneurysmal perimesencephalic hemorrhage, spontaneous rebleeding does not occur. The lack of reported recurrences may lead to less cautious administration of antithrombotic therapy. METHODS: Case report. RESULTS: A 57-year-old woman with a perimesencephalic pattern of hemorrhage and negative CT angiography was treated with Carbasalate calcium and intravenous heparin because of an acute coronary syndrome. Three days after installment of this antithrombotic therapy she experienced a recurrent perimesencephalic hemorrhage leading to hydrocephalus and a decrease in consciousness. She died the same day as a result of ventricular fibrillation. CONCLUSION: In the early phase after perimesencephalic hemorrhage, anticoagulant therapy may lead to rebleeding. The risks and benefits of antithrombotic therapy should be carefully weighed in patients with a perimesencephalic pattern of hemorrhage and negative CT angiography.
[Purulent pericarditis in a patient with knee pain].[Pubmed:18664217]
Ned Tijdschr Geneeskd. 2008 Jun 14;152(24):1382-6.
A 40-year-old man with pain in his left, swollen knee that persisted for 6 weeks presented with chest pain, dyspnoea and subfebrile temperature. The pain worsened during inspiration and was relieved by sitting up straight. The electrocardiogram showed pericarditis. The patient was treated with high-dose Carbasalate calcium. Initially, echocardiography revealed a 2-cm pericardial effusion with no signs of influx inhibition. Blood cultures were positive for Neisseria meningitidis, and treatment was expanded to include antibiotics. Based on a deterioration in patient condition and the tamponade image, pericardiocentesis was performed. Repeated transoesophageal echocardiography showed insufficient drainage of the purulent pericardial effusion. Pericardiectomy was then performed. The patient was doing very well, 3 years after this. If left untreated, the mortality rate for purulent pericarditis approaches 100%. It is therefore important to diagnose at an early stage so that treatment with antibiotics and surgery, which can reduce mortality considerably, can be performed.
[Periorbital swelling caused by carbasalate calcium].[Pubmed:15366727]
Ned Tijdschr Geneeskd. 2004 Jul 31;148(31):1550-4.
A 61-year-old man presented with visual problems due to a marked two-sided periorbital swelling that had started three years earlier. There was no obvious underlying cause and he had persistent rhinoconjunctival symptoms. At the time the swelling started he was already suffering from xanthelasmata palpebrarum and chronic obstructive pulmonary disease. He had been taking simvastatin and salmeterol-fluticason for a year and Carbasalate calcium for two years. There was marked periorbital swelling accompanied by xanthelasmata palpebrarum and slight chemosis and erythema of the eyelids. After exclusion of other potential causes for the swelling, acenocoumarol was prescribed in place of Carbasalate calcium. This resulted in a quick recovery from the swelling and rhinoconjunctival symptoms. Provocation with acetyl salicylic acid led to renewed swelling of both eyelids. Periorbital angio-oedema, a relatively uncommonly reported side effect of acetyl salicylic acid and its derivates, can occur even after previous long-term medication use.
What is the lowest dose of aspirin for maximum suppression of in vivo thromboxane production after a transient ischemic attack or ischemic stroke?[Pubmed:15026612]
Cerebrovasc Dis. 2004;17(4):296-302.
BACKGROUND: There is still worldwide disagreement about the optimal lowest dose of aspirin to be used in patients after a transient ischemic attack (TIA) or nondisabling stroke. We measured the urinary 11-dehydro-thromboxane-B(2) (uTXB(2)) excretion to compare the degree of suppression of in vivo platelet activation by various low doses of aspirin. METHODS: 60 patients were randomly allocated to treatment with either 30, 50, 75 or 325 mg of aspirin. All patients received a 413-mg loading dose of Carbasalate calcium (equivalent to 325 mg of aspirin) on day 0. The study population was stratified into a subgroup with acute ischemic stroke (AIS; n = 20; onset of symptoms <48 h) and a subgroup with a recent TIA or minor stroke (TIA/mS; n = 40) with onset of symptoms beyond 30 days, but less than a year previously. Urine samples were collected on day 0, 1, 5, 11 and 28 in patients with AIS, and on day 0, 11 and 28 in the patients with a TIA/mS. RESULTS: On day 28, mean uTXB(2) levels were 241, 130, 217 and 187 pmol/mmol creatinine in the four treatment groups (ANOVA, p = 0.43). In the AIS subgroup, uTXB(2) remained suppressed on days 5 and 11 in all except the patients with the lowest dose (mean uTXB(2) on days 5 and 11: 475 and 392 pmol/mmol creatinine; log-transformed ANOVA, p = 0.05). CONCLUSION: In patients with a TIA or nondisabling stroke, a daily dose of 30 mg of aspirin provides sufficient suppression of thromboxane synthesis. No indication of a dose-effect relationship was found. However, whether such a low dose adequately suppresses thromboxane synthesis in patients with acute stroke is uncertain.


