IsoflavoneCAS# 574-12-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 574-12-9 | SDF | Download SDF |
| PubChem ID | 72304 | Appearance | Powder |
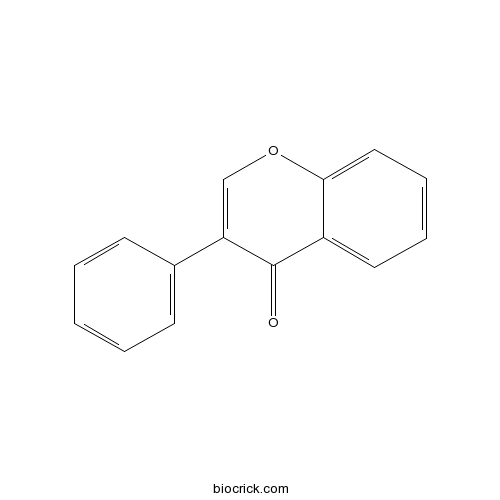
| Formula | C15H10O2 | M.Wt | 222.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 3-Phenylchromone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3-phenylchromen-4-one | ||
| SMILES | C1=CC=C(C=C1)C2=COC3=CC=CC=C3C2=O | ||
| Standard InChIKey | GOMNOOKGLZYEJT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O2/c16-15-12-8-4-5-9-14(12)17-10-13(15)11-6-2-1-3-7-11/h1-10H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Isoflavone Dilution Calculator

Isoflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4996 mL | 22.4982 mL | 44.9964 mL | 89.9928 mL | 112.491 mL |
| 5 mM | 0.8999 mL | 4.4996 mL | 8.9993 mL | 17.9986 mL | 22.4982 mL |
| 10 mM | 0.45 mL | 2.2498 mL | 4.4996 mL | 8.9993 mL | 11.2491 mL |
| 50 mM | 0.09 mL | 0.45 mL | 0.8999 mL | 1.7999 mL | 2.2498 mL |
| 100 mM | 0.045 mL | 0.225 mL | 0.45 mL | 0.8999 mL | 1.1249 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Benzoin ethyl ether
Catalog No.:BCC8855
CAS No.:574-09-4
- Oroxin A
Catalog No.:BCN1202
CAS No.:57396-78-8
- Irsogladine
Catalog No.:BCC4562
CAS No.:57381-26-7
- Isochlorogenic acid C
Catalog No.:BCN2498
CAS No.:57378-72-0
- Bombinakinin-GAP
Catalog No.:BCC5903
CAS No.:573671-91-7
- Dihydroepistephamiersine 6-acetate
Catalog No.:BCN5775
CAS No.:57361-74-7
- Tacalcitol
Catalog No.:BCC1975
CAS No.:57333-96-7
- Congo Red
Catalog No.:BCC8023
CAS No.:573-58-0
- Liriodendrin
Catalog No.:BCN5774
CAS No.:573-44-4
- Boehmenan
Catalog No.:BCN5773
CAS No.:57296-22-7
- Ridaforolimus (Deforolimus, MK-8669)
Catalog No.:BCC4605
CAS No.:572924-54-0
- Boc-D-Phe(4-F)-OH
Catalog No.:BCC3218
CAS No.:57292-45-2
- Fraxetin
Catalog No.:BCN5903
CAS No.:574-84-5
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
- 8-O-Acetylshanzhiside methyl ester
Catalog No.:BCN5776
CAS No.:57420-46-9
- (2S)-2alpha-(1,3-Benzodioxol-5-yl)-3,5-dihydro-5alpha-methoxy-3beta-methyl-5-allyl-2H-benzofuran-6-one
Catalog No.:BCN6606
CAS No.:57430-03-2
- Methylergometrine maleate
Catalog No.:BCC6691
CAS No.:57432-61-8
- Resiniferatoxin
Catalog No.:BCC6951
CAS No.:57444-62-9
- p-Menth-8-ene-1,2-diol
Catalog No.:BCN5777
CAS No.:57457-97-3
- 2-Epi-3a-epiburchellin
Catalog No.:BCN7013
CAS No.:57457-99-5
- Stigmasta-3,5-diene
Catalog No.:BCC8254
CAS No.:4970-37-0
- H-Asn-OMe.HCl
Catalog No.:BCC2876
CAS No.:57461-34-4
- Ibuprofen Lysine
Catalog No.:BCC2547
CAS No.:57469-77-9
- 16-Deoxysaikogenin F
Catalog No.:BCN6414
CAS No.:57475-62-4
Isolation, Synthesis, and Antisepsis Effects of a C-Methylcoumarinochromone Isolated from Abronia nana Cell Culture.[Pubmed:29762033]
J Nat Prod. 2018 May 25;81(5):1173-1182.
Only a few Isoflavones have been isolated from plants of the genus Abronia. The biological properties of compounds isolated from Abronia species have not been well established, and their antisepsis effects have not been reported yet. In the present study, a new C-methylcoumarinochromone, was isolated from Abronia nana suspension cultures. Its structure was deduced as 9,11-dihydroxy-10-methylcoumarinochromone (boeravinone Y, 1) by spectroscopic data analysis and verified by chemical synthesis. The potential inhibitory effects of 1 against high mobility group box 1 (HMGB1)-mediated septic responses were investigated. Results showed that 1 effectively inhibited lipopolysaccharide-induced release of HMGB1 and suppressed HMGB1-mediated septic responses, in terms of reduction of hyperpermeability, leukocyte adhesion and migration, and cell adhesion molecule expression. In addition, 1 increased the phagocytic activity of macrophages and exhibited bacterial clearance effects in the peritoneal fluid and blood of mice with cecal ligation and puncture-induced sepsis. Collectively, these results suggested that 1 might have potential therapeutic activity against various severe vascular inflammatory diseases via inhibition of the HMGB1 signaling pathway.
The O-methylated isoflavone, formononetin, inhibits human ovarian cancer cell proliferation by sub G0/G1 cell phase arrest through PI3K/AKT and ERK1/2 inactivation.[Pubmed:29761845]
J Cell Biochem. 2018 Sep;119(9):7377-7387.
Formononetin is an Isoflavone that is extracted from red clovers or soy. It has anti-oxidant, anti-proliferative, and anti-tumor effects against cells in various diseases. Several cohort studies have indicated that phytoestrogen intake, including formononetin, could reduce the risk of various carcinogenesis. In fact, many case-control studies have indicated the potential value of flavonoids as drug supplements in the treatment of many cancer patients. However, the toxic effects and the anti-cancer mechanism of formononetin in ovarian cancer are unknown. We investigated the toxicological mechanism of formononetin in ES2 and OV90 ovarian cancer cells. Formononetin suppressed cell proliferation through sub G0/G1 phase arrest and increased apoptosis in both cell lines. Furthermore, it induced loss of mitochondrial membrane potential and generation of reactive oxygen species in ES2 and OV90 cells. The formononetin-mediated regulation of cell proliferation and apoptosis involved decreased phosphorylation of ERK1/2, P90RSK, AKT, P70S6K, and S6 proteins, and increased phosphorylation of P38 protein in ES2 and OV90 cells. Co-treatment of formononetin with pharmacological inhibitors (LY294002 or U0126) revealed additional anti-proliferative effects on the two human ovarian cancer cell types. Conclusively, the results indicate the potential value of formononetin as an anti-cancer agent in human ovarian cancer.
Role of Oxidative Stress in Diabetic Retinopathy and the Beneficial Effects of Flavonoids.[Pubmed:29766782]
Curr Pharm Des. 2018;24(19):2180-2187.
Diabetic Retinopathy (DR) is one of the leading causes of decreased vision and blindness in developed countries. Diabetes-induced metabolic disorder is believed to increase oxidative stress in the retina. This results in deleterious change through dysregulation of cellular physiology that damages both neuronal and vascular cells. In this review, we first highlight the evidence of potential metabolic sources and pathways which increase oxidative stress that contribute to retinal pathology in diabetes. As oxidative stress is a central factor in the pathophysiology of DR, antioxidants therapy would be beneficial towards preventing the retinal damage. A number of experimental studies by our group and others showed that dietary flavonoids cause reduction in increased oxidative stress and other beneficial effects in diabetic retina. We then discuss the beneficial effects of the six major flavonoid families, such as flavanones, flavanols, flavonols, Isoflavones, flavones and anthocyanins, which have been studied to improve retinal damage. Flavanoids, being known antioxidants, may ameliorate the retinal degenerative factors including apoptosis, inflammation and neurodegeneration in diabetes. Therefore, intake of potential dietary flavonoids would limit oxidative stress and thereby prevent the retinal damage, and subsequently the development of DR.
Short-Term Soy Protein Isolate Feeding Prevents Liver Steatosis and Reduces Serum ALT and AST Levels in Obese Female Zucker Rats.[Pubmed:29757972]
Biomedicines. 2018 May 14;6(2). pii: biomedicines6020055.
Non-alcoholic fatty liver disease is a common liver disorder worldwide and is associated with obesity. We investigated effects of obesity and short-term intake of soy protein with Isoflavones (SPI) on body weight change, energy intake, liver steatosis, and serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and leptin levels. Seventeen lean and seventeen obese (fa/fa) female Zucker rats were randomly assigned to either casein or SPI diet for 8 weeks. Body weight was recorded twice weekly; feed intake was measured weekly. Livers were examined histologically, and serum AST, ALT, and leptin levels were measured. Obese soy-fed (OS) rats gained more weight but had lower liver steatosis than obese casein-fed (OC) rats. Energy intake for OS versus OC rats were only different at weeks 2 and 3. Serum AST and ALT levels were lower in OS versus OC rats. Obesity increased serum leptin levels for both diets. In summary, short-term SPI intake reduced liver steatosis, and the only time points at which the mean energy intakes of OS and OC rats differed were at weeks 2 and 3, where OS rats had a higher mean energy intake, which may have accounted for the increased body weight in OS rats.
EB 2017 Article: Soy protein isolate feeding does not result in reproductive toxicity in the pre-pubertal rat testis.[Pubmed:29763383]
Exp Biol Med (Maywood). 2018 May;243(8):695-707.
The Isoflavone phytoestrogens found in the soy protein isolate used in soy infant formulas have been shown to have estrogenic actions in the developing male reproductive tract resulting in reproductive toxicity. However, few studies have examined potential estrogenicity of soy protein isolate as opposed to that of pure Isoflavones. In this study, we fed weanling male Sprague-Dawley rats a semi-purified diet with casein or soy protein isolate as the sole protein source from postnatal day 21 to 33. Additional groups were fed casein or soy protein isolate and treated s.c. with 10 microg/kg/d estradiol via osmotic minipump. Estradiol treatment reduced testis, prostate weights, and serum androgen concentrations ( P < 0.05). Soy protein isolate had no effect. Estradiol up-regulated 489 and down-regulated 1237 testicular genes >1.5-fold ( P < 0.05). In contrast, soy protein isolate only significantly up-regulated expression of 162 genes and down-regulated 16 genes. The top 30 soy protein isolate-up-regulated genes shared 93% concordance with estradiol up-regulated genes. There was little overlap between soy protein isolate down-regulated genes and those down-regulated by estradiol treatment. Functional annotation analysis revealed significant differences in testicular biological processes affected by estradiol or soy protein isolate. Estradiol had major actions on genes involved in reproductive processes including down-regulation of testicular steroid synthesis and expression of steroid receptor activated receptor (Star) and cytochrome P450 17alpha-hydroxylase/(Cyp17a1). In contrast, soy protein isolate primarily affected pathways associated with macromolecule modifications including ubiquitination and histone methylation. Our results indicate that rather than acting as a weak estrogen in the developing testis, soy protein isolate appears to act as a selective estrogen receptor modulator with little effect on reproductive processes. Impact statement Soy protein isolate (SPI) is the sole protein used to make soy-based infant formulas. SPI contains phytoestrogens, which are structurally similar to estradiol. These phytoestrogens, daidzein, genistein, and equol, fit the definition of endocrine-disrupting compounds, and at high concentrations, have estrogenic actions resulting in reproductive toxicity in the developing male, when provided as isolated chemicals. However, few animal studies have examined the potential estrogenicity of SPI as opposed to pure Isoflavones. In this study, SPI feeding did not elicit an estrogenic response in the testis nor any adverse outcomes including reduced testicular growth, or androgen production during early development in rats when compared to those receiving estradiol. These findings are consistent with emerging data showing no differences in reproductive development in males and female children that received breast milk, cow's milk formula, or soy infant formula during the postnatal feeding period.
Flavonoid subclasses and type 2 diabetes mellitus risk: a meta-analysis of prospective cohort studies.[Pubmed:29768032]
Crit Rev Food Sci Nutr. 2018 May 16:1-13.
Epidemiological studies have suggested controversial associations between flavonoid subclasses and type 2 diabetes mellitus (T2DM) risk. The aim of the present meta-analysis was to quantitatively estimate these associations with prospective cohort study. A systematic literature search in PubMed and Scopus databases was performed up to May 2018. Multivariate-adjust relative risks (RRs) with corresponding 95% confidence intervals (CIs) for the highest versus the lowest category were pooled by using a random-effects model. Using restricted cubic spline regression model, non-linear dose-response analysis was estimated. Nine independent prospective cohort studies with 172,058 participants and 16910 events were included. Dietary intakes of flavanols, flavonols, flavan-3-ols and Isoflavones were inversely associated with T2DM risk, and the summary RRs were 0.86 (95%CI: 0.77, 0.97), 0.91 (95%CI: 0.85, 0.98), 0.90 (95%: 0.82, 0.99) and 0.91 (95%CI: 0.84, 0.98), respectively. Dose-response analysis showed that 135 mg/day increment of flavanols (95%CI: 0.92, 0.96; P for trend <0.001), 50 mg/day increment of flavonols (95%CI: 0.88, 0.99, P for trend = 0.021), 68 mg/day increment of flavan-3-ols (95%CI: 0.92, 0.96, P for trend <0.001), or 1.8 mg/day increment of Isoflavones (95%CI: 0.92, 0.97, P for trend <0.001) were associated with 6% reduction in T2DM risk. Non-significant association was observed with respect to flavanones and flavones. The present meta-analysis provides substantial evidence that dietary intakes of flavanols, flavonols, flavan-3-ols and Isoflavones were inversely associated with T2DM risk, respectively. Higher dietary intakes of flavanol-, flavonol-, flavan-3-ol- and Isoflavone-foods would have beneficial effects for protection against T2DM.


