GMQ hydrochlorideSelective ASIC3 opener CAS# 5361-15-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5361-15-9 | SDF | Download SDF |
| PubChem ID | 12253739 | Appearance | Powder |
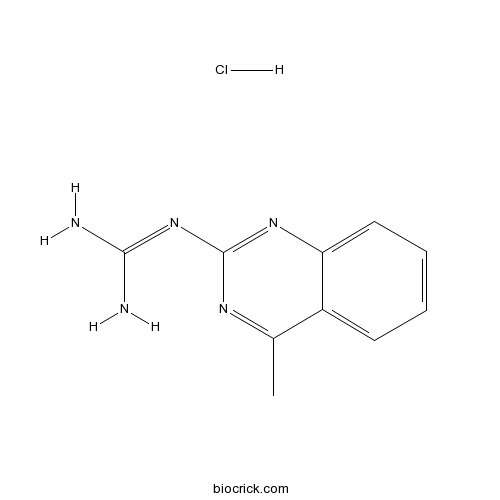
| Formula | C10H12ClN5 | M.Wt | 237.69 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 5 mM in water with gentle warming | ||
| Chemical Name | 2-(4-methylquinazolin-2-yl)guanidine;hydrochloride | ||
| SMILES | CC1=NC(=NC2=CC=CC=C12)N=C(N)N.Cl | ||
| Standard InChIKey | VNPZWQRWOHFAPD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H11N5.ClH/c1-6-7-4-2-3-5-8(7)14-10(13-6)15-9(11)12;/h2-5H,1H3,(H4,11,12,13,14,15);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective opener of acid-sensing ion channel 3 (ASIC3) at pH 7.4. Alters the pH dependence of ASIC1a and ASIC1b; does not affect the activation curve of ASIC2a. |

GMQ hydrochloride Dilution Calculator

GMQ hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.2072 mL | 21.0358 mL | 42.0716 mL | 84.1432 mL | 105.179 mL |
| 5 mM | 0.8414 mL | 4.2072 mL | 8.4143 mL | 16.8286 mL | 21.0358 mL |
| 10 mM | 0.4207 mL | 2.1036 mL | 4.2072 mL | 8.4143 mL | 10.5179 mL |
| 50 mM | 0.0841 mL | 0.4207 mL | 0.8414 mL | 1.6829 mL | 2.1036 mL |
| 100 mM | 0.0421 mL | 0.2104 mL | 0.4207 mL | 0.8414 mL | 1.0518 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 1-Hydroxyrutaecarpine
Catalog No.:BCN5709
CAS No.:53600-24-1
- Perillyl alcohol
Catalog No.:BCN3876
CAS No.:536-59-4
- Ethionamide
Catalog No.:BCC3778
CAS No.:536-33-4
- 5'-Iodoresiniferatoxin
Catalog No.:BCC7031
CAS No.:535974-91-5
- Secolongifolenediol
Catalog No.:BCN6989
CAS No.:53587-37-4
- H-Tyr-OBzl.TosOH
Catalog No.:BCC3124
CAS No.:53587-11-4
- 5-Galloylquinic acid
Catalog No.:BCN3060
CAS No.:53584-43-3
- Beta-Amyrenonol acetate
Catalog No.:BCN4620
CAS No.:5356-56-9
- (-)-Bicuculline methochloride
Catalog No.:BCN3763
CAS No.:53552-05-9
- Diaveridine
Catalog No.:BCC8936
CAS No.:5355-16-8
- Benzoyl-L-histidine
Catalog No.:BCC8866
CAS No.:5354-94-9
- Apigenin 7-O-methylglucuronide
Catalog No.:BCN5708
CAS No.:53538-13-9
- Teucvin
Catalog No.:BCN8375
CAS No.:53625-15-3
- Vindesine
Catalog No.:BCN2607
CAS No.:53643-48-4
- Dehydrobruceantin
Catalog No.:BCN7617
CAS No.:53662-98-9
- 4,6-Dimethoxy-2H-1-benzopyran-2-one
Catalog No.:BCN3452
CAS No.:53666-78-7
- O-1918
Catalog No.:BCC7313
CAS No.:536697-79-7
- ICI 89406
Catalog No.:BCC6807
CAS No.:53671-71-9
- QX 222
Catalog No.:BCC6906
CAS No.:5369-00-6
- QX 314 chloride
Catalog No.:BCC7326
CAS No.:5369-03-9
- 3-Acetyl-5-Hydroxymethyl-7-Hydroxycoumarin
Catalog No.:BCC9201
CAS No.:53696-74-5
- Diperodon HCl
Catalog No.:BCC3766
CAS No.:537-12-2
- Tropacocaine
Catalog No.:BCN1934
CAS No.:537-26-8
- Isoporoidine
Catalog No.:BCN1890
CAS No.:537-28-0
ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain.[Pubmed:21143836]
Mol Pain. 2010 Dec 8;6:88.
BACKGROUND: Acid-sensing ion channels (ASICs) have long been known to sense extracellular protons and contribute to sensory perception. Peripheral ASIC3 channels represent natural sensors of acidic and inflammatory pain. We recently reported the use of a synthetic compound, 2-guanidine-4-methylquinazoline (GMQ), to identify a novel nonproton sensing domain in the ASIC3 channel, and proposed that, based on its structural similarity with GMQ, the arginine metabolite agmatine (AGM) may be an endogenous nonproton ligand for ASIC3 channels. RESULTS: Here, we present further evidence for the physiological correlation between AGM and ASIC3. Among arginine metabolites, only AGM and its analog arcaine (ARC) activated ASIC3 channels at neutral pH in a sustained manner similar to GMQ. In addition to the homomeric ASIC3 channels, AGM also activated heteromeric ASIC3 plus ASIC1b channels, extending its potential physiological relevance. Importantly, the process of activation by AGM was highly sensitive to mild acidosis, hyperosmolarity, arachidonic acid (AA), lactic acid and reduced extracellular Ca2+. AGM-induced ASIC3 channel activation was not through the chelation of extracellular Ca2+ as occurs with increased lactate, but rather through a direct interaction with the newly identified nonproton ligand sensing domain. Finally, AGM cooperated with the multiple inflammatory signals to cause pain-related behaviors in an ASIC3-dependent manner. CONCLUSIONS: Nonproton ligand sensing domain might represent a novel mechanism for activation or sensitization of ASIC3 channels underlying inflammatory pain-sensing under in vivo conditions.


