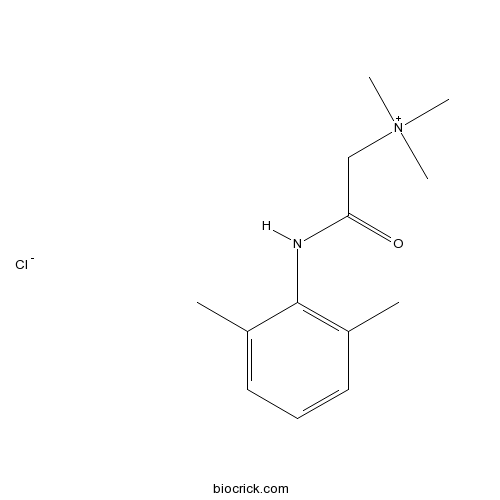
QX 222Na+ channel blocker CAS# 5369-00-6 |
- TCS 359
Catalog No.:BCC1183
CAS No.:301305-73-7
- Tandutinib (MLN518)
Catalog No.:BCC4499
CAS No.:387867-13-2
- Amuvatinib (MP-470, HPK 56)
Catalog No.:BCC2258
CAS No.:850879-09-3
- TG101209
Catalog No.:BCC2198
CAS No.:936091-14-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 5369-00-6 | SDF | Download SDF |
| PubChem ID | 9795082 | Appearance | Powder |
| Formula | C13H21ClN2O | M.Wt | 256.77 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | [2-(2,6-dimethylanilino)-2-oxoethyl]-trimethylazanium;chloride | ||
| SMILES | CC1=C(C(=CC=C1)C)NC(=O)C[N+](C)(C)C.[Cl-] | ||
| Standard InChIKey | WFKXSWWTOZBDME-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H20N2O.ClH/c1-10-7-6-8-11(2)13(10)14-12(16)9-15(3,4)5;/h6-8H,9H2,1-5H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sodium channel blocker. |

QX 222 Dilution Calculator

QX 222 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8945 mL | 19.4727 mL | 38.9454 mL | 77.8907 mL | 97.3634 mL |
| 5 mM | 0.7789 mL | 3.8945 mL | 7.7891 mL | 15.5781 mL | 19.4727 mL |
| 10 mM | 0.3895 mL | 1.9473 mL | 3.8945 mL | 7.7891 mL | 9.7363 mL |
| 50 mM | 0.0779 mL | 0.3895 mL | 0.7789 mL | 1.5578 mL | 1.9473 mL |
| 100 mM | 0.0389 mL | 0.1947 mL | 0.3895 mL | 0.7789 mL | 0.9736 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ICI 89406
Catalog No.:BCC6807
CAS No.:53671-71-9
- O-1918
Catalog No.:BCC7313
CAS No.:536697-79-7
- 4,6-Dimethoxy-2H-1-benzopyran-2-one
Catalog No.:BCN3452
CAS No.:53666-78-7
- Dehydrobruceantin
Catalog No.:BCN7617
CAS No.:53662-98-9
- Vindesine
Catalog No.:BCN2607
CAS No.:53643-48-4
- Teucvin
Catalog No.:BCN8375
CAS No.:53625-15-3
- GMQ hydrochloride
Catalog No.:BCC6351
CAS No.:5361-15-9
- 1-Hydroxyrutaecarpine
Catalog No.:BCN5709
CAS No.:53600-24-1
- Perillyl alcohol
Catalog No.:BCN3876
CAS No.:536-59-4
- Ethionamide
Catalog No.:BCC3778
CAS No.:536-33-4
- 5'-Iodoresiniferatoxin
Catalog No.:BCC7031
CAS No.:535974-91-5
- Secolongifolenediol
Catalog No.:BCN6989
CAS No.:53587-37-4
- QX 314 chloride
Catalog No.:BCC7326
CAS No.:5369-03-9
- 3-Acetyl-5-Hydroxymethyl-7-Hydroxycoumarin
Catalog No.:BCC9201
CAS No.:53696-74-5
- Diperodon HCl
Catalog No.:BCC3766
CAS No.:537-12-2
- Tropacocaine
Catalog No.:BCN1934
CAS No.:537-26-8
- Isoporoidine
Catalog No.:BCN1890
CAS No.:537-28-0
- Convolvine
Catalog No.:BCN1904
CAS No.:537-30-4
- Chlorophorin
Catalog No.:BCN3288
CAS No.:537-41-7
- Pterostilbene
Catalog No.:BCN2539
CAS No.:537-42-8
- N-Acetyl-L-tyrosine
Catalog No.:BCC9082
CAS No.:537-55-3
- Soyasaponin Be Methyl Ester
Catalog No.:BCN5925
CAS No.:117210-13-6
- N-Acetyl-m-toluidine
Catalog No.:BCC9083
CAS No.:537-92-8
- beta-Amyrenonol methylthiomethyl ether
Catalog No.:BCN3354
CAS No.:
Coapplication of lidocaine and membrane-impermeable lidocaine derivative QX-222 produces divergent effects on evoked and spontaneous nociceptive behaviors in mice.[Pubmed:25506595]
Biomed Res Int. 2014;2014:628729.
The present study was aimed at investigating the analgesic properties of a combination of lidocaine and QX-222 and its effects on evoked pain behavior (complete Freund's adjuvant-induced allodynia and hyperalgesia in inflammatory condition) and spontaneous pain behavior (formalin-induced acute pain) in mice. Drugs were injected adjacent to sciatic nerve or into plantar. Motor function, thermal withdrawal latency, mechanical withdrawal threshold, and licking/biting were evaluated by behavioral tests. A combination of lidocaine and QX-222 adjacent sciatic nerve injection produced the long-lasting sensory-specific nerve block, and intraplantar injection inhibited spontaneous pain in the formalin-treated mice but did not detectably attenuated hyperalgesia and allodynia in the complete Freund's adjuvant- (CFA-) treated mice. Our results suggest that a combination of lidocaine and QX-222 achieves a long-lasting differential block (sensory selective) and produces divergent effects on evoked and spontaneous pain behaviors in mice.
Cooperative interactions between general anesthetics and QX-222 within the pore of the acetylcholine receptor ion channel.[Pubmed:7520126]
Mol Pharmacol. 1994 Jul;46(1):169-75.
To test the hypothesis that general anesthetics block nicotinic acetylcholine receptor channels by binding within the pore of the channel, we looked for competitive interactions between ether and QX-222 at the single channel current level. Experiments were performed on outside-out patches excised from BC3H-1 cells. QX-222 causes channels to flicker as it repeatedly binds within the pore of the channel and blocks the flow of current through the channel. Ether reduces the apparent unitary conductance of the channel. This effect of ether may be due to frequent, short-lived, unresolved, blockages of the channel. When both ether and QX-222 are applied, the effects of both drugs are seen on single channels. However, the duration of QX-222 blocking events are longer when ether is present; the duration of block is 0.89 +/- 0.06 ms with 30 microM QX-222 alone and 2.23 +/- 0.37 ms with 30 microM QX-222 + 20 mM ether (n = 5 +/- S.D.; -100 mV). Similar results are obtained when butanol is used in place of ether. We conclude that ether and QX-222 do not compete for a common binding site. Conversely, ether decreases the dissociation rate of QX-222. The simplest interpretation of these data is that the binding sites for ether and the aromatic moiety of QX-222 are distinct but close to each other; when ether is bound to its site, the binding of QX-222 is stabilized. We cannot, however, discount the possibility that ether stabilizes QX-222 by binding to a remote site and allosterically modifying the pore of the channel.
The voltage dependence of recovery from use-dependent block by QX-222 separates mechanisms for drug egress in the cardiac sodium channel.[Pubmed:16637111]
Biochem Pharmacol. 2006 Apr 28;71(9):1299-1307.
In neuronal sodium channels of squid giant axons, recovery from QX-222 block is slowed by hyperpolarization. However, in ventricular cells, hyperpolarization speeds recovery. Previously, we showed that isoform-specific residues in the external side of the cardiac sodium channel isoform (D1P-loop C373 and D4S6 T1752) influence use-dependent block (UDB) by lidocaine. To determine whether these isoformspecific residues contribute to the contrasting voltage-dependent recovery observed in ventricular myocytes, we measured recovery rates from UDB by QX-222 at holding potentials of 120, 140, 160 and 180 mV for wild-type cardiac channel (WT), the mutants C373Y (CY) and T1752V (TV), and C373Y/T1752V (CY/TV). Unlike neuronal channels, cardiac sodium channels recovered from QX block faster at hyperpolarized potentials. All mutations slowed QX-222 recovery, with the greatest rate reduction observed for the double mutant, indicating that the isoform-specific residues define external drug paths. The recovery rates varied linearly with voltage over the range tested, and we used the slopes of rate versus voltage plots to quantify voltage dependence. The TV mutation caused reduction in recovery rates without changing the slope, indicating that the mutation closed a voltage-independent egress path. The CY mutation, however, flattened the slope and reduced the voltage dependence of recovery. In addition, the reduction in rate caused by CY/TV is less than the sum of those for CY and TV, suggesting that the impacts of these two residues are interrelated. Therefore, we propose that the isoform-specific residues C373 and T1752 change recovery from UDB by distinct mechanisms but determine a common drug egress path.
Blockade of lysophosphatidylcholine-modified cardiac Na channels by a lidocaine derivative QX-222.[Pubmed:8770124]
Am J Physiol. 1996 Aug;271(2 Pt 2):H790-7.
Single Na channels from rat and rabbit ventricular cells were studied with use of the excised inside-out patch-clamp technique. To investigate local anesthetic interactions with Na channels modified by the ischemic metabolite lysophosphatidylcholine (LPC), the quaternary ammonium lidocaine derivative QX-222 [2-(trimethylamino)-N-(2,6-dimethylphenyl)acetamide] was applied to the cytoplasmic side of patches from untreated cells and from those treated with LPC for approximately 1 h. Single-channel amplitudes and kinetics for unmodified channels were similar to those reported previously for cardiac cells with a single-component, mean-channel open time. LPC-modified channels showed prolonged open channel bursting with a two-component, mean open time, suggesting two open states. Conductance sublevels to the 60-70% level of the main conductance were found in both unmodified and LPC-modified channels and also with and without QX-222 present. QX-222 reversibly shortened the open time of the unmodified channel and for both open times of the LPC-modified channel without decreasing single-channel amplitude. Calculated association rates for QX-222 with the channel were found to be greater for the open states of the modified channel than those for the unmodified channel. Thus the lidocaine analogue QX-222 interacts with and blocks the open state of both unmodified and LPC-modified, cardiac Na channels. The blocking effect on LPC-modified channels would be predicted to be greater both because of the longer dwell time in the high-affinity open states for modified channels and also because of an intrinsically greater association rate in the modified channels.
Local anaesthetic blockade of neuronal nicotinic ACh receptor-channels in rat parasympathetic ganglion cells.[Pubmed:7517326]
Br J Pharmacol. 1994 Mar;111(3):663-72.
1 The effects of the local anaesthetics QX-222 and procaine on nicotinic acetylcholine (ACh)-evoked currents in cultured parasympathetic cardiac neurones of the rat were investigated by use of the whole-cell, perforated-patch, and outside-out recording configurations of the patch clamp method. 2 QX-222 and procaine, applied to the extracellular surface, reversibly inhibited the peak amplitude of the whole-cell nicotinic ACh-evoked current in a concentration-dependent manner, with half-maximal inhibitory concentrations (IC50) of 28 microM and 2.8 microM, respectively, at -80 mV. In these neurones, the sustained inward current mediated by M1 muscarinic receptor activation was unaltered by QX-222, and neither local anaesthetic affected the adenosine 5'-triphosphate (ATP)-evoked current. 3 QX-222 and procaine block of nicotinic ACh-evoked inward current was voltage-dependent and enhanced by hyperpolarization. An e-fold change in their dissociation equilibrium constants (Kd) resulted from a 62 mV and a 122 mV change in membrane potential, respectively. 4 Both local anaesthetics produce a concentration-dependent increase in the half-time of decay of the nicotinic ACh-evoked inward current. 5 Measurements of unitary currents in outside-out patches showed that QX-222 reversibly increased the mean burst duration and closed time and reduced the mean channel open time and open-state probability of the nicotinic ACh receptor-channel (AChR) in a concentration-dependent manner. 6 The Kd and voltage sensitivity of local anaesthetic block of the nicotinic AChR in rat intracardiac neurones suggests that the pore-forming region of this channel differs from that of the AChR in frog and rat skeletal muscle and from the neuronal alpha 4 beta 2 ACh receptor-channel.


