Benzoyl-L-histidineCAS# 5354-94-9 |
Quality Control & MSDS
Number of papers citing our products

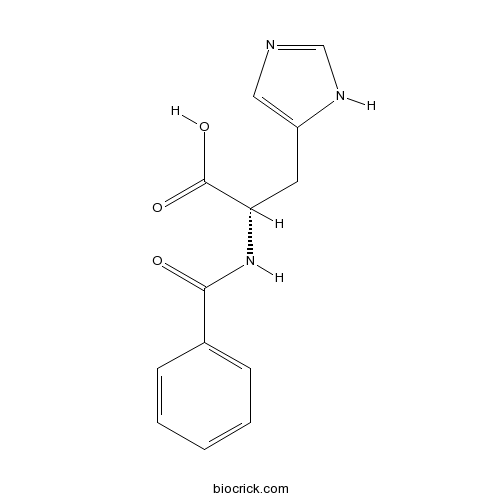
Chemical structure

3D structure
| Cas No. | 5354-94-9 | SDF | Download SDF |
| PubChem ID | 152263 | Appearance | Powder |
| Formula | C13H13N3O3 | M.Wt | 259.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-benzamido-3-(1H-imidazol-5-yl)propanoic acid | ||
| SMILES | C1=CC=C(C=C1)C(=O)NC(CC2=CN=CN2)C(=O)O | ||
| Standard InChIKey | AUDPUFBIVWMAED-NSHDSACASA-N | ||
| Standard InChI | InChI=1S/C13H13N3O3/c17-12(9-4-2-1-3-5-9)16-11(13(18)19)6-10-7-14-8-15-10/h1-5,7-8,11H,6H2,(H,14,15)(H,16,17)(H,18,19)/t11-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Benzoyl-L-histidine Dilution Calculator

Benzoyl-L-histidine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.8565 mL | 19.2827 mL | 38.5654 mL | 77.1307 mL | 96.4134 mL |
| 5 mM | 0.7713 mL | 3.8565 mL | 7.7131 mL | 15.4261 mL | 19.2827 mL |
| 10 mM | 0.3857 mL | 1.9283 mL | 3.8565 mL | 7.7131 mL | 9.6413 mL |
| 50 mM | 0.0771 mL | 0.3857 mL | 0.7713 mL | 1.5426 mL | 1.9283 mL |
| 100 mM | 0.0386 mL | 0.1928 mL | 0.3857 mL | 0.7713 mL | 0.9641 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Apigenin 7-O-methylglucuronide
Catalog No.:BCN5708
CAS No.:53538-13-9
- Luteolin-3-O-beta-D-glucuronide
Catalog No.:BCN3826
CAS No.:53527-42-7
- Durantoside I
Catalog No.:BCN4621
CAS No.:53526-67-3
- Durantoside II
Catalog No.:BCN4622
CAS No.:53526-66-2
- 7-Amino-4-(Trifluoromethyl)Coumarin
Catalog No.:BCC9208
CAS No.:53518-15-3
- 4,9,9'-Trihydroxy-3,3'-dimethoxy-8,4'-oxyneolignan
Catalog No.:BCN1425
CAS No.:53505-68-3
- PIT 1
Catalog No.:BCC7873
CAS No.:53501-41-0
- Marein
Catalog No.:BCN6477
CAS No.:535-96-6
- Trigonelline
Catalog No.:BCN2371
CAS No.:535-83-1
- Pipecolinic acid
Catalog No.:BCN8150
CAS No.:535-75-1
- Apohyoscine
Catalog No.:BCN1870
CAS No.:535-26-2
- 4-Methoxycinnamyl alcohol
Catalog No.:BCN7515
CAS No.:53484-50-7
- Diaveridine
Catalog No.:BCC8936
CAS No.:5355-16-8
- (-)-Bicuculline methochloride
Catalog No.:BCN3763
CAS No.:53552-05-9
- Beta-Amyrenonol acetate
Catalog No.:BCN4620
CAS No.:5356-56-9
- 5-Galloylquinic acid
Catalog No.:BCN3060
CAS No.:53584-43-3
- H-Tyr-OBzl.TosOH
Catalog No.:BCC3124
CAS No.:53587-11-4
- Secolongifolenediol
Catalog No.:BCN6989
CAS No.:53587-37-4
- 5'-Iodoresiniferatoxin
Catalog No.:BCC7031
CAS No.:535974-91-5
- Ethionamide
Catalog No.:BCC3778
CAS No.:536-33-4
- Perillyl alcohol
Catalog No.:BCN3876
CAS No.:536-59-4
- 1-Hydroxyrutaecarpine
Catalog No.:BCN5709
CAS No.:53600-24-1
- GMQ hydrochloride
Catalog No.:BCC6351
CAS No.:5361-15-9
- Teucvin
Catalog No.:BCN8375
CAS No.:53625-15-3
Synthesis of six epoxyketooctadecenoic acid (EKODE) isomers, their generation from nonenzymatic oxidation of linoleic acid, and their reactivity with imidazole nucleophiles.[Pubmed:17979284]
J Org Chem. 2007 Dec 7;72(25):9471-80.
As a class of linoleic acid oxidation products, epoxyketooctadecenoic acids (EKODEs), are formed in vivo and in vitro by a free radical mechanism initiated by either enzymatic or nonenzymatic pathways. They have so far been made available in small-scale quantities, often as isomeric mixtures, from reductive decomposition of linoleic acid-derived hydroperoxides. There is major interest in these compounds owing to their highly potent biological activities and their ability to covalently modify proteins. The synthesis of six EKODE regio- and stereoisomers, two trans alpha',beta'-epoxy-alpha,beta-enones, and two trans and the two cis gamma,delta,-epoxy-alpha,beta-enones was accomplished, with the key steps being Wittig-type reactions and aldol condensations. All six EKODE isomers were confirmed by HPLC to be generated in the autoxidation of linoleic acid promoted by Fe(II)/ascorbic acid through spiking in of authentic samples. On the basis of evidence for EKODE modification of protein His residues, the reactions of Nalpha-Benzoyl-L-histidine with autoxidizing linoleic acid and with the individual EKODE isomers were compared, as were the kinetics of the various EKODE reactions with imidazole nucleophiles. The structures of His-EKODE-(E)-I adducts were confirmed to reflect conjugate addition (epoxide ring remains intact) through an NMR study of the reaction of imidazole with a generic EKODE-(E)-I analog. The synthesis of the EKODE isomers makes these important molecules available for further chemical and biological evaluation.
Detoxications in peripatus. Sulphate, phosphate and histidine conjugations.[Pubmed:5472152]
Biochem J. 1970 Jun;118(1):1-8.
Phenols were detoxified in the Onycophoran Peripatoides novaezealandiae by conjugation with sulphuric acid and phosphoric acid, but no evidence for a glycoside detoxication could be found. [(14)C]Benzoic acid was metabolized in 24h to N(2)-Benzoyl-L-histidine, which was identified by electrophoresis, chromatography and dilution analysis. Similar conjugates were formed with p-aminobenzoic acid and p-nitrobenzoic acid. In longer-duration experiments further unidentified metabolites were formed, two of which appeared to result from the further metabolism of the histidine conjugate.


