DL-TBOAinhibitor of excitatory amino acid transporters CAS# 205309-81-5 |
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- TFB-TBOA
Catalog No.:BCC5919
CAS No.:480439-73-4
- Dihydrokainic acid
Catalog No.:BCC6556
CAS No.:52497-36-6
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- LDN 212320
Catalog No.:BCC6361
CAS No.:894002-50-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 205309-81-5 | SDF | Download SDF |
| PubChem ID | 5311218 | Appearance | Powder |
| Formula | C11H13NO5 | M.Wt | 239.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 5 mM in water with gentle warming | ||
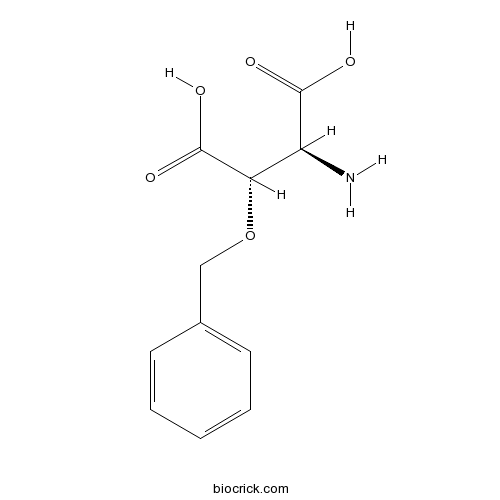
| Chemical Name | (2S,3S)-2-amino-3-phenylmethoxybutanedioic acid | ||
| SMILES | C1=CC=C(C=C1)COC(C(C(=O)O)N)C(=O)O | ||
| Standard InChIKey | BYOBCYXURWDEDS-IUCAKERBSA-N | ||
| Standard InChI | InChI=1S/C11H13NO5/c12-8(10(13)14)9(11(15)16)17-6-7-4-2-1-3-5-7/h1-5,8-9H,6,12H2,(H,13,14)(H,15,16)/t8-,9-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A competitive, non-transportable blocker of excitatory amino acid transporters (IC50 values are 70, 6, and 6 μM for EAAT1, EAAT2 and EAAT3 respectively). Also inhibits EAAT4 and EAAT5 (Ki values are 4.4 and 3.2 μM respectively). Displays high selectivity for EAATs over ionotropic and metabotropic glutamate receptors. Also available as part of the Excitatory Amino Acid Transporter Inhibitor. |

DL-TBOA Dilution Calculator

DL-TBOA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1801 mL | 20.9004 mL | 41.8008 mL | 83.6016 mL | 104.5019 mL |
| 5 mM | 0.836 mL | 4.1801 mL | 8.3602 mL | 16.7203 mL | 20.9004 mL |
| 10 mM | 0.418 mL | 2.09 mL | 4.1801 mL | 8.3602 mL | 10.4502 mL |
| 50 mM | 0.0836 mL | 0.418 mL | 0.836 mL | 1.672 mL | 2.09 mL |
| 100 mM | 0.0418 mL | 0.209 mL | 0.418 mL | 0.836 mL | 1.045 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ki: 42 μM for EAAT1; 5.7 μM for EAAT2
Glutamate acts as an excitatory neurotransmitter in the mammalian central nervous system and a potent neurotoxin. Glutamate transporters also play an important role in maintaining the extracellular glutamate concentration below neurotoxic levels and therefore contribute to the prevention of neuronal damage. DL-TBOA was synthesized and examined as an inhibitor of sodium-dependent glutamate/aspartate transporters(excitatory amino acid transporters).
In vitro: DL-TBOA inhibited the uptake of [14C]glutamate in COS-1 cells overexpressing the human excitatory amino acid transporter-1 (EAAT1) (Ki = 42 μM) with almost the same potency as DL-threo-b-hydroxyaspartate (Ki = 58 μM). With regard to the human excitatory amino acid transporter-2 (EAAT2), the inhibitory effect of DL-TBOA (Ki = 5.7 μM) was much more potent than that of dihydrokainate (Ki = 79 μM), which is well known as a selective blocker of this subtype. [1].
In vivo: Microdialysis administration of 500 μM DL-TBOA into the hippocampus increased 3.4- and nine-fold the extracellular levels of aspartate and glutamate, respectively. Upon stereotaxic administration it induced neuronal damage dose-dependently in CA1 and dentate gyrus, and convulsive behavior. Electroencephalographic recording showed limbic seizures appearance in the hippocampus after DL-TBOA infusion. [2].
Clinical trial: Up to now, DL-TBOA is still in the preclinical development stage.
Reference:
[1] Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998 Feb;53(2):195-201.
[2] Montiel T, Camacho A, Estrada-Sánchez AM, Massieu L. Differential effects of the substrate inhibitor l-trans-pyrrolidine-2,4-dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience. 2005;133(3):667-78.
- Isonardoperoxide
Catalog No.:BCN7628
CAS No.:205248-65-3
- 2'-MeCCPA
Catalog No.:BCC7311
CAS No.:205171-12-6
- (+)-Peusedanol
Catalog No.:BCC9119
CAS No.:20516-23-8
- Lansiumarin C
Catalog No.:BCN4895
CAS No.:205115-75-9
- Lansiumarin A
Catalog No.:BCN4894
CAS No.:205115-73-7
- Talatisamine
Catalog No.:BCN5403
CAS No.:20501-56-8
- 3,7,4'-Trihydroxy-5-methoxy-8-prenylflavanone
Catalog No.:BCN1503
CAS No.:204935-85-3
- Curzerenone
Catalog No.:BCN3009
CAS No.:20493-56-5
- Nardosinonediol
Catalog No.:BCN8118
CAS No.:20489-11-6
- Procumbide
Catalog No.:BCN3932
CAS No.:20486-27-5
- 8-O-Acetyltorilolone
Catalog No.:BCN7094
CAS No.:20482-21-7
- Fmoc-Lys(ivDde)-OH
Catalog No.:BCC3520
CAS No.:204777-78-6
- Amifostine
Catalog No.:BCC5232
CAS No.:20537-88-6
- Pinocembrin 7-O-(3'-galloyl-4',6'-(S)-hexahydroxydiphenoyl)-beta-D-glucose
Catalog No.:BCN6769
CAS No.:205370-59-8
- BU 224 hydrochloride
Catalog No.:BCC6765
CAS No.:205437-64-5
- Taxezopidine G
Catalog No.:BCN6947
CAS No.:205440-22-8
- Paederoside
Catalog No.:BCN3437
CAS No.:20547-45-9
- Rauvovertine B
Catalog No.:BCN7726
CAS No.:2055073-72-6
- Rauvovertine C
Catalog No.:BCN7727
CAS No.:2055073-74-8
- Rauvovertine A
Catalog No.:BCN7728
CAS No.:2055073-75-9
- Fmoc-D-Phe(3-Cl)-OH
Catalog No.:BCC3170
CAS No.:205526-23-4
- Fmoc-D-Phe(4-I)-OH
Catalog No.:BCC3262
CAS No.:205526-29-0
- Fenfangjine G
Catalog No.:BCN3297
CAS No.:205533-81-9
- 2'',4''-Di-O-(Z-p-coumaroyl)afzelin
Catalog No.:BCN4676
CAS No.:205534-17-4
Neurotoxic and neuroprotective effects of the glutamate transporter inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) during physiological and ischemia-like conditions.[Pubmed:12742081]
Neurochem Int. 2003 Sep-Oct;43(4-5):371-80.
Maintenance of low extracellular glutamate ([Glu](O)) preventing excitotoxic cell death requires fast removal of glutamate from the synaptic cleft. This clearance is mainly provided by high affinity sodium-dependent glutamate transporters. These transporters can, however, also be reversed and release glutamate to the extracellular space in situations with energy failure. In this study the cellular localisation of the glutamate transporters GLAST and GLT-1 in organotypic hippocampal slice cultures was studied by immunofluorescence confocal microscopy, under normal culture conditions, and after a simulated ischemic insult, achieved by oxygen and glucose deprivation (OGD). In accordance with in vivo findings, GLAST and GLT-1 were primarily expressed by astrocytes under normal culture conditions, but after OGD some damaged neurons also expressed GLAST and GLT-1. The potential damaging effect of inhibition of the glutamate transporters by DL-threo-beta-benzyloxyaspartate (DL-TBOA) was studied using cellular uptake of propidium iodide (PI) as a quantitative marker for the cell death. Addition of DL-TBOA for 48 h was found to induce significant cell death in all hippocampal regions, with EC(50) values ranging from 38 to 48 microM for the different hippocampal subregions. The cell death was prevented by addition of the glutamate receptor antagonists NBQX and MK-801, together with an otherwise saturating concentration of DL-TBOA (100 microM). Finally, the effect of inhibition of glutamate release, via reverse operating transporters during OGD, was investigated. Addition of a sub-toxic (10 microM) dose of DL-TBOA during OGD, but not during the subsequent 48 h recovery period, significantly reduced the OGD-induced PI uptake. It is concluded: (1) that the cellular expression of the glutamate transporters GLAST and GLT-1 in hippocampal slice cultures in general corresponds to the expression in vivo, (2) that inhibition of the glutamate transporters induces cell death in the slice cultures, and (3) that partial inhibition during simulation of ischemia by OGD protects against the induced PI uptake, most likely by blocking the reverse operating transporters otherwise triggered by the energy failure.
Differential effects of the substrate inhibitor l-trans-pyrrolidine-2,4-dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo.[Pubmed:15890455]
Neuroscience. 2005;133(3):667-78.
The extracellular concentration of glutamate is highly regulated by transporter proteins, due to its neurotoxic properties. Dysfunction or reverse activation of these transporters is related to the extracellular accumulation of excitatory amino acids and neuronal damage associated with ischemia and hypoglycemia. We have investigated by microdialysis the effects of the substrate and the non-substrate inhibitors of glutamate transporters, l-trans-2,4-pyrrolidine dicarboxylate (PDC) and DL-threo-beta-benzyloxyaspartate (DL-TBOA), respectively, on the extracellular levels of amino acids in the rat hippocampus in vivo. In addition, we have studied the effect of both inhibitors on neuronal damage after direct administration into the hippocampus and striatum. Electroencephalographic activity was recorded after the intrahippocampal infusion of DL-TBOA or PDC. Microdialysis administration of 500 microM DL-TBOA into the hippocampus increased 3.4- and nine-fold the extracellular levels of aspartate and glutamate, respectively. Upon stereotaxic administration it induced neuronal damage dose-dependently in CA1 and dentate gyrus, and convulsive behavior. Electroencephalographic recording showed the appearance of limbic seizures in the hippocampus after DL-TBOA infusion. In the striatum it also induced dose-dependent neuronal damage. These effects were prevented by the i.p. administration of the glutamate receptor antagonists (+)-5-methyl-10,11-dihydroxy-5H-dibenzo(a,d)cyclohepten-5,10-iminemaleate and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)-quinoxaline. In contrast to DL-TBOA, PDC (500 microM) induced a more discrete elevation of excitatory amino acids levels (2.6- and three-fold in aspartate and glutamate, respectively), no neuronal damage or behavioral changes, and no alterations in electroencephalographic activity. The differential results obtained with DL-TBOA and PDC might be attributed to their distinct effects on the extracellular concentration of amino acids. Results are relevant to the understanding of the role of glutamate transporters in amino acid removal or release and the induction of excitotoxic cell death.
Characterization of depolarization-coupled release of glutamate from cultured mouse cerebellar granule cells using DL-threo-beta-benzyloxyaspartate (DL-TBOA) to distinguish between the vesicular and cytoplasmic pools.[Pubmed:12742087]
Neurochem Int. 2003 Sep-Oct;43(4-5):417-24.
Release of preloaded [3H]D-aspartate in response to depolarization induced by N-methyl-D-aspartate (NMDA) or the endogenous agonist glutamate was characterized using cultured glutamatergic cerebellar granule neurons. Release from the vesicular and the cytoplasmic glutamate pools, respectively, was distinguished employing the competitive, non-transportable glutamate transport inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA). NMDA (300 microM)-induced release was enhanced (50%) by a simultaneous elevation of the extracellular potassium concentration to 15 mM, which lifts the voltage-dependent magnesium block of the NMDA receptors. This NMDA/K(+)-induced release was not sensitive to DL-TBOA (100 microM) but was inhibited by 75% in the presence of the unspecific calcium channel antagonist La(3+) (100 microM). Glutamate (100 microM) induced a large fractional release of the preloaded [3H]D-aspartate and in the presence of DL-TBOA the release was reduced by approximately 50%. In contrast, release evoked by 25 microM glutamate was not inhibited by DL-TBOA. These results indicate that the release elicited by 100 microM glutamate is comprised of a significant glutamate transporter-mediated component in addition to the vesicular release while the NMDA/K(+)-induced release is vesicular in nature. It is likely that the high glutamate concentration (100 microM) may facilitate heteroexchange of the preloaded [3H]D-aspartate.
The glutamate transport inhibitor DL-Threo-beta-Benzyloxyaspartic acid (DL-TBOA) differentially affects SN38- and oxaliplatin-induced death of drug-resistant colorectal cancer cells.[Pubmed:25981639]
BMC Cancer. 2015 May 16;15:411.
BACKGROUND: Colorectal cancer (CRC) is a leading cause of cancer death globally and new biomarkers and treatments are severely needed. METHODS: Here, we employed HCT116 and LoVo human CRC cells made resistant to either SN38 or oxaliplatin, to investigate whether altered expression of the high affinity glutamate transporters Solute Carrier (SLC)-1A1 and -1A3 (EAAT3, EAAT1) is associated with the resistant phenotypes. Analyses included real-time quantitative PCR, immunoblotting and immunofluorescence analyses, radioactive tracer flux measurements, and biochemical analyses of cell viability and glutathione content. Results were evaluated using one- and two-way ANOVA and Students two-tailed t-test, as relevant. RESULTS: In SN38-resistant HCT116 and LoVo cells, SLC1A1 expression was down-regulated ~60 % and up-regulated ~4-fold, respectively, at both mRNA and protein level, whereas SLC1A3 protein was undetectable. The changes in SLC1A1 expression were accompanied by parallel changes in DL-Threo-beta-Benzyloxyaspartic acid (TBOA)-sensitive, UCPH101-insensitive [(3)H]-D-Aspartate uptake, consistent with increased activity of SLC1A1 (or other family members), yet not of SLC1A3. DL-TBOA co-treatment concentration-dependently augmented loss of cell viability induced by SN38, while strongly counteracting that induced by oxaliplatin, in both HCT116 and LoVo cells. This reflected neither altered expression of the oxaliplatin transporter Cu(2+)-transporter-1 (CTR1), nor changes in cellular reduced glutathione (GSH), although HCT116 cell resistance per se correlated with increased cellular GSH. DL-TBOA did not significantly alter cellular levels of p21, cleaved PARP-1, or phospho-Retinoblastoma protein, yet altered SLC1A1 subcellular localization, and reduced chemotherapy-induced p53 induction. CONCLUSIONS: SLC1A1 expression and glutamate transporter activity are altered in SN38-resistant CRC cells. Importantly, the non-selective glutamate transporter inhibitor DL-TBOA reduces chemotherapy-induced p53 induction and augments CRC cell death induced by SN38, while attenuating that induced by oxaliplatin. These findings may point to novel treatment options in treatment-resistant CRC.
Effects of threo-beta-hydroxyaspartate derivatives on excitatory amino acid transporters (EAAT4 and EAAT5).[Pubmed:11677257]
J Neurochem. 2001 Oct;79(2):297-302.
D,L-threo-beta-Benzyloxyaspartate (D,L-TBOA), an analog of threo-beta-hydroxyaspartate (THA) possessing a bulky substituent, is a potent non-transportable blocker for the excitatory amino acid transporters, EAAT1, 2 and 3, while L-threo-beta-methoxyaspartate (L-TMOA) is a blocker for EAAT2, but a substrate for EAAT1 and EAAT3. To characterize the actions of these THA analogs and the function of EAAT4 and EAAT5, we performed electrophysiological analyses in EAAT4 or EAAT5 expressed on Xenopus oocytes. In EAAT4-expressing oocytes, D,L-TBOA acted as a non-transportable blocker, while L-TMOA like D,L-THA was a competitive substrate. In contrast, D,L-THA, D,L-TBOA and L-TMOA all strongly attenuated the glutamate-induced currents generated by EAAT5. Among them, L-TMOA showed the most potent inhibitory action. Moreover, D,L-THA, D,L-TBOA and L-TMOA themselves elicited outward currents at negative potentials and remained inward at positive potentials suggesting that D,L-TBOA and L-TMOA, as well as D,L-THA, not only act as non-transportable blockers, but also block the EAAT5 leak currents. These results indicate that EAATs 4 and 5 show different sensitivities to THA analogs although they share properties of a glutamate-gated chloride channel.
Syntheses of optically pure beta-hydroxyaspartate derivatives as glutamate transporter blockers.[Pubmed:11078189]
Bioorg Med Chem Lett. 2000 Nov 6;10(21):2407-10.
DL-threo-beta-benzyloxyaspartate (DL-TBOA) is a non-transportable blocker of the glutamate transporters that serves as an indispensable tool for the investigation of the physiological roles of the transporters. To examine the precise interaction between a blocker and the transporters, we synthesized the optically pure isomers (L- and D-TBOA) and its erythro-isomers. L-TBOA is the most potent blocker for the human excitatory amino acid transporters (EAAT1-3), while D-TBOA revealed a difference in the pharmacophores between EAAT1 and EAAT3. We also synthesized the substituent variants (methyl or naphthylmethyl derivatives) of L-TBOA. The results obtained here suggest that bulky substituents are crucial for non-transportable blockers.
Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin.[Pubmed:10411944]
Proc Natl Acad Sci U S A. 1999 Jul 20;96(15):8733-8.
Maintaining glutamate at low extracellular concentrations in the central nervous system is necessary to protect neurons from excitotoxic injury and to ensure a high signal-to-noise ratio for glutamatergic synaptic transmission. We have used DL-threo-beta-benzyloxyaspartate (TBOA), an inhibitor of glutamate uptake, to determine the role of glutamate transporters in the regulation of extracellular glutamate concentration. By using the N-methyl-D-aspartate receptors of patched CA3 hippocampal neurons as "glutamate sensors," we observed that application of TBOA onto organotypic hippocampal slices led to a rapid increase in extracellular glutamate concentration. This increase was Ca(2+)-independent and was observed in the presence of tetrodotoxin. Moreover, prevention of vesicular glutamate release with clostridial toxins did not affect the accumulation of glutamate when uptake was inhibited. Inhibition of glutamine synthase, however, increased the rate of accumulation of extracellular glutamate, indicating that glial glutamate stores can serve as a source in this process. TBOA blocked synaptically evoked transporter currents in astrocytes without inducing a current mediated by the glutamate transporter. This indicates that this inhibitor is not transportable and does not release glutamate by heteroexchange. These results show that under basal conditions, the activity of glutamate transporters compensates for the continuous, nonvesicular release of glutamate from the intracellular compartment. As a consequence, acute disruption of transporter activity immediately results in significant accumulation of extracellular glutamate.
DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters.[Pubmed:9463476]
Mol Pharmacol. 1998 Feb;53(2):195-201.
DL-threo-beta-Benzyloxyaspartate (DL-TBOA), a novel derivative of DL-threo-beta-hydroxyaspartate, was synthesized and examined as an inhibitor of sodium-dependent glutamate/aspartate (excitatory amino acid) transporters. DL-TBOA inhibited the uptake of [14C]glutamate in COS-1 cells expressing the human excitatory amino acid transporter-1 (EAAT1) (Ki = 42 microM) with almost the same potency as DL-threo-beta-hydroxyaspartate (Ki = 58 microM). With regard to the human excitatory amino acid transporter-2 (EAAT2), the inhibitory effect of DL-TBOA (Ki = 5.7 microM) was much more potent than that of dihydrokainate (Ki = 79 microM), which is well known as a selective blocker of this subtype. Electrophysiologically, DL-TBOA induced no detectable inward currents in Xenopus laevis oocytes expressing human EAAT1 or EAAT2. However, it significantly reduced the glutamate-induced currents, indicating the prevention of transport. The dose-response curve of glutamate was shifted by adding DL-TBOA without a significant change in the maximum current. The Kb values for human EAAT1 and EAAT2 expressed in X. laevis oocytes were 9.0 microM and 116 nM, respectively. These results demonstrated that DL-TBOA is, so far, the most potent competitive blocker of glutamate transporters. DL-TBOA did not show any significant effects on either the ionotropic or metabotropic glutamate receptors. Moreover, DL-TBOA is chemically much more stable than its benzoyl analog, a previously reported blocker of excitatory amino acid transporters; therefore, DL-TBOA should be a useful tool for investigating the physiological roles of transporters.


