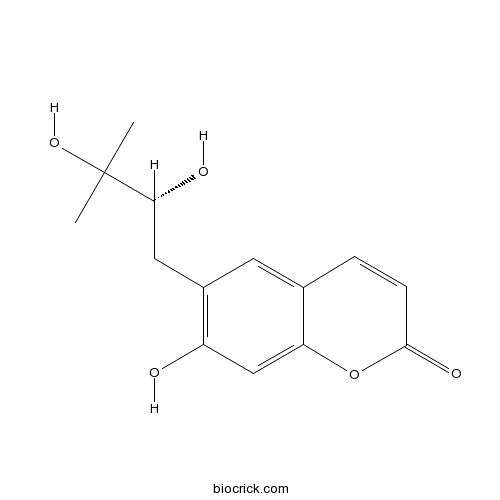
(+)-PeusedanolCAS# 20516-23-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 20516-23-8 | SDF | Download SDF |
| PubChem ID | 15296614 | Appearance | White powder |
| Formula | C14H16O5 | M.Wt | 264.3 |
| Type of Compound | Phenylpropanes | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO and methanol; insoluble in water | ||
| Chemical Name | 6-[(2R)-2,3-dihydroxy-3-methylbutyl]-7-hydroxychromen-2-one | ||
| SMILES | CC(C)(C(CC1=C(C=C2C(=C1)C=CC(=O)O2)O)O)O | ||
| Standard InChIKey | WRTWKAQFZYXAEJ-GFCCVEGCSA-N | ||
| Standard InChI | InChI=1S/C14H16O5/c1-14(2,18)12(16)6-9-5-8-3-4-13(17)19-11(8)7-10(9)15/h3-5,7,12,15-16,18H,6H2,1-2H3/t12-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

(+)-Peusedanol Dilution Calculator

(+)-Peusedanol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7836 mL | 18.9179 mL | 37.8358 mL | 75.6716 mL | 94.5895 mL |
| 5 mM | 0.7567 mL | 3.7836 mL | 7.5672 mL | 15.1343 mL | 18.9179 mL |
| 10 mM | 0.3784 mL | 1.8918 mL | 3.7836 mL | 7.5672 mL | 9.4589 mL |
| 50 mM | 0.0757 mL | 0.3784 mL | 0.7567 mL | 1.5134 mL | 1.8918 mL |
| 100 mM | 0.0378 mL | 0.1892 mL | 0.3784 mL | 0.7567 mL | 0.9459 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Lansiumarin C
Catalog No.:BCN4895
CAS No.:205115-75-9
- Lansiumarin A
Catalog No.:BCN4894
CAS No.:205115-73-7
- Talatisamine
Catalog No.:BCN5403
CAS No.:20501-56-8
- 3,7,4'-Trihydroxy-5-methoxy-8-prenylflavanone
Catalog No.:BCN1503
CAS No.:204935-85-3
- Curzerenone
Catalog No.:BCN3009
CAS No.:20493-56-5
- Nardosinonediol
Catalog No.:BCN8118
CAS No.:20489-11-6
- Procumbide
Catalog No.:BCN3932
CAS No.:20486-27-5
- 8-O-Acetyltorilolone
Catalog No.:BCN7094
CAS No.:20482-21-7
- Fmoc-Lys(ivDde)-OH
Catalog No.:BCC3520
CAS No.:204777-78-6
- Olcegepant
Catalog No.:BCC1818
CAS No.:204697-65-4
- Epitaraxerol
Catalog No.:BCN4677
CAS No.:20460-33-7
- Talnetant hydrochloride
Catalog No.:BCC1982
CAS No.:204519-66-4
- 2'-MeCCPA
Catalog No.:BCC7311
CAS No.:205171-12-6
- Isonardoperoxide
Catalog No.:BCN7628
CAS No.:205248-65-3
- DL-TBOA
Catalog No.:BCC5735
CAS No.:205309-81-5
- Amifostine
Catalog No.:BCC5232
CAS No.:20537-88-6
- Pinocembrin 7-O-(3'-galloyl-4',6'-(S)-hexahydroxydiphenoyl)-beta-D-glucose
Catalog No.:BCN6769
CAS No.:205370-59-8
- BU 224 hydrochloride
Catalog No.:BCC6765
CAS No.:205437-64-5
- Taxezopidine G
Catalog No.:BCN6947
CAS No.:205440-22-8
- Paederoside
Catalog No.:BCN3437
CAS No.:20547-45-9
- Rauvovertine B
Catalog No.:BCN7726
CAS No.:2055073-72-6
- Rauvovertine C
Catalog No.:BCN7727
CAS No.:2055073-74-8
- Rauvovertine A
Catalog No.:BCN7728
CAS No.:2055073-75-9
- Fmoc-D-Phe(3-Cl)-OH
Catalog No.:BCC3170
CAS No.:205526-23-4
The alpha-amylase and alpha-glucosidase inhibitory activities of the dichloromethane extracts and constituents of Ferulago bracteata roots.[Pubmed:29233045]
Pharm Biol. 2018 Dec;56(1):18-24.
CONTEXT: Ferulago (Apiaceae) species have been used since ancient times for the treatment of intestinal worms, hemorrhoids, and as a tonic, digestive, aphrodisiac, or sedative, as well as in salads or as a spice due to their special odors. OBJECTIVES: This study reports the alpha-amylase and alpha-glucosidase inhibitory activities of dichloromethane extract and bioactive compounds isolated from Ferulago bracteata Boiss. & Hausskn. roots. MATERIALS AND METHODS: The isolated compounds obtained from dichloromethane extract of Ferulago bracteata roots through bioassay-guided fractionation and isolation process were evaluated for their in vitro alpha-amylase and alpha-glucosidase inhibitory activities at 5000-400 microg/mL concentrations. Compound structures were elucidated by detailed analyses (NMR and MS). RESULTS: A new coumarin, peucedanol-2'-benzoate (1), along with nine known ones, osthole (2), imperatorin (3), bergapten (4), prantschimgin (5), grandivitinol (6), suberosin (7), xanthotoxin (8), felamidin (9), umbelliferone (10), and a sterol mixture consisted of stigmasterol (11), beta-sitosterol (12) was isolated from the roots of F. bracteata. Felamidin and suberosin showed significant alpha-glucosidase inhibitory activity (IC50 0.42 and 0.89 mg/mL, respectively) when compared to the reference standard acarbose (IC50 4.95 mg/mL). However, none of the tested extracts were found to be active on alpha-amylase inhibition. DISCUSSION AND CONCLUSIONS: The present study demonstrated that among the compounds isolated from CH2Cl2 fraction of F. bracteata roots, coumarins were determined as the main chemical constituents of this fraction. This is the first report on isolation and characterization of the bioactive compounds from root extracts of F. bracteata and on their alpha-amylase and alpha-glucosidase inhibitory activities.
Simultaneous analysis of seven marker compounds from Saposhnikoviae Radix, Glehniae Radix and Peucedani Japonici Radix by HPLC/PDA.[Pubmed:27016947]
Arch Pharm Res. 2016 May;39(5):695-704.
A new combination of high performance liquid chromatography (HPLC) method coupled with photodiode array (PDA) analysis has been developed for the simultaneous quantitative determination of seven major components in Saposhnikoviae Radix (SR), Glehniae Radix (GR) and Peucedani Japonici Radix (PR), namely peucedanol 7-O-beta-D-glucopyranoside (1), prim-O-glucosylcimifugin (2), cimifugin (3), 4'-O-beta-D-glucosyl-5-O-methylvisamminol (4), bergapten (5), sec-O-glucosylhamaudol (6), and imperatorin (7). Clear separation of these seven components were achieved on a Phenomenex Kinetex C18 (250 x 4.6 mm, 5 mum) column by gradient elution of water (A) and methanol (B) as mobile phase. The flow rate was 1.0 mL/min and the UV detector wavelength was set at 254 nm. The method was successfully used in the analysis of SR, GR, and PR with relatively simple conditions and procedures, and the results were satisfactory for linearity, recovery, precision, accuracy, stability and robustness. The results indicate that the established HPLC/PDA method is suitable for the classification of SR, GR, and PR.
Transport of Twelve Coumarins from Angelicae Pubescentis Radix across a MDCK-pHaMDR Cell Monolayer-An in Vitro Model for Blood-Brain Barrier Permeability.[Pubmed:26121397]
Molecules. 2015 Jun 25;20(7):11719-32.
Angelicae Pubescentis Radix (APR), a widely used traditional Chinese medicine, is reported to have central nervous system activities. The purpose of this study was to characterize the blood-brain barrier permeability of twelve coumarins from APR including umbelliferone (1), osthol (2), scopoletin (3), peucedanol (4), ulopterol (5), angepubebisin (6), psoralen (7), xanthotoxin (8), bergapten (9), isoimperatorin (10), columbianadin (11), and columbianetin acetate (12) with an in vitro model using a MDCK-pHaMDR cell monolayer. The cell monolayer was validated to be suitable for the permeation experiments. The samples' transports were analyzed by high performance liquid chromatography and their apparent permeability coefficients (Papp) were calculated. According to the Papp value, most coumarins could be characterized as well-absorbed compounds except for 4, 10 and 11 which were moderately absorbed ones, in concentration-dependent and time-dependent manners. The results of P-glycoprotein (P-gp) inhibitor (verapamil) experiments showed that the transport of coumarin 4 was affected by the transport protein P-gp. Sigmoid functions between permeability log(Papp AP-BL*MW0.5) and log D (at pH 7.4) were established to analyze the structure-activity relationship of coumarins. The results provide useful information for discovering the substance basis for the central nervous system activities of APR, and predicting the permeability of other coumarins through BBB.
[Study on the coumarin glucosides of Angelica dahurica].[Pubmed:23627089]
Zhong Yao Cai. 2012 Nov;35(11):1785-8.
OBJECTIVE: To study the coumarin glucosides of Angelica dahurica. METHODS: Fresh roots and rhizomes of Angelica dahurica were extracted with ethanol at room temperature. Repeated column chromatography and preparative HPLC were used to isolate and purify the compounds. Their structures were elucidated on the basis of chemical evidence and spectral analysis. RESULTS: Twenty-six coumarin glucosides were isolated from Angelica dahurica, while here we reported 7 of them: sec.-O-beta-D-Galactopyranosyl-(R)-byakangelicin (I);8-O-beta-D-Galactopyranosyl-xanthotoxol (II); 7-O-beta-D-Apiofuranosyl-(1-->6)-beta-D-Glucopyranosyl-peucedanol (III); (R)-peucedanol-7-O-beta-D-Glucopyranoside (IV); sec.-O-beta-D-Glucopyranosyl-(R)-Oxypeucedaninhydrate (V); 7-O-beta-D-Galactopyranosyl-Sco-poletin( VI); Aesculin (VII). CONCLUSION: Compound V is a new compound, Compound VII is isolated from Umbellifera for the first time, compounds III, IV are isolated from Angelica for the first time,while compound I, II and VI are isolated from this plant for the first time.
Novel coumarin and furan from the roots of Angelica pubescens f. biserrata.[Pubmed:20183310]
J Asian Nat Prod Res. 2009 Aug;11(8):698-703.
A new natural coumarin, angepubebisin (1), and a new furan, angepubefurin (2), together with the five known compounds, umbelliferone, angelol B (3), ulopterol (4), peucedanol (5), and scopoletin, were isolated from the roots of Angelica pubescens Maxim. f. biserrata Shan et Yuan. The structures of angepubebisin (1) and known compounds were determined by spectroscopic methods, including IR, UV, EI-MS, HR-FTICR-MS, 1D-, and 2D-NMR spectral analyses, and angepubefurin (2) was determined by HR-FTICR-MS and X-ray diffraction analyses.
Neuroprotective coumarins from the root of Angelica gigas: structure-activity relationships.[Pubmed:18087802]
Arch Pharm Res. 2007 Nov;30(11):1368-73.
An n-butanol-soluble fraction of the root of Angelica gigas Nakai (Umbelliferae) exhibited significant protection against glutamate-induced toxicity in primary cultured rat cortical cells. Using neuroprotective activity-guided fractionation, nine coumarins; marmesinin (1), nodakenin (2), columbianetin-O-beta-D-glucopyranoside (3), (S)-peucedanol-7-O-beta-D-glucopyranoside (4), (S)-peucedanol-3'-O-beta-D-glucopyranoside (5), skimmin (6), apiosylskimmin (7), isoapiosylskimmin (8) and magnolioside (9), were isolated from the n-butanol fraction. Of these nine coumarins, three dihydrofuranocoumarins; 1, 2 and 3, exhibited significant neuroprotective activities against glutamate-induced toxicity, exhibiting cell viabilities of about 50% at concentrations ranging from 0.1 to 10 microM. To explore the structure-activity relationships of coumarins, sixteen previously isolated compounds; 10-25, were simultaneously evaluated in the same system. Our results revealed that cyclization of the isoprenyl group, such as dihydropyran or dihydrofuran, or the furan ring at the C-6 position of coumarin, as well as lipophilicity played an important role in the neuroprotective activity of coumarins.
Antidiabetic coumarin and cyclitol compounds from Peucedanum japonicum.[Pubmed:15646792]
Arch Pharm Res. 2004 Dec;27(12):1207-10.
The antidiabetic activity-guided fractionation and isolation of the 80% EtOH extracts from Peucedani Radix (Peucedanum japonicum, Umbelliferae) led to the isolation and characterization of a coumarin and a cyclitol as active principles, that is, peucedanol 7-O-beta-D-glucopyranoside (1) and myo-inositol (2). Their structures were identified by spectroscopic methods. Compound 1 showed 39% inhibition of postprandial hyperglycemia at 5.8 mg/kg dose, and compound 2 also significantly inhibited postprandial hyperglycemia by 34% (P<0.05).
Antioxidant compounds from the leaves of Peucedanum japonicum thunb.[Pubmed:12926867]
J Agric Food Chem. 2003 Aug 27;51(18):5255-61.
Seventeen compounds were isolated from the n-butanol soluble fraction of the leaves of Peucedanum japonicum Thunb. On the basis of MS and various NMR spectroscopic techniques, the structures of the isolated compounds were determined as isoquercitrin (1), rutin (2), 3-O-caffeoylquinic acid (3), 4-O-caffeoylquinic acid (4), 5-O-caffeoylquinic acid (5), cnidioside A (6), praeroside II (7), praeroside III (8), apterin (9), esculin (10), (R)-peucedanol (11), (R)-peucedanol 7-O-beta-d-glucopyranoside (12), l-tryptophan (13), uracil (14), guanosine (15), uridine (16), and thymidine (17). All compounds except 11 and 12 were isolated for the first time from P. japonicum. Several isolated compounds were quantified by high-performance liquid chromatography analysis. In addition, all isolated compounds were examined for radical scavenging on 1,1-diphenyl-2-picrylhydrazyl radical and for inhibition of oxidation of liposome induced by 2,2'-azobis(2-amidinopropane)dihydrochloride. Compounds 2-5 were found to be the major potent constituents, which contribute to the antioxidant activity of P. japonicum leaves.
Medicinal foodstuffs. XX. Vasorelaxant active constituents from the roots of Angelica furcijuga Kitagawa: structures of hyuganins A, B, C, and D.[Pubmed:11045445]
Chem Pharm Bull (Tokyo). 2000 Oct;48(10):1429-35.
From the methanolic extract with vasorelaxant activity obtained from Angelica furcijuga Kitagawa, four new khellactone-type coumarins, hyuganins A, B, C, and D, were isolated together with twelve known coumarins, two known acetylenic compounds, and a known lignan. The structures of hyuganins A, B, C, and D were determined on the basis of chemical and physicochemical evidence. Nine principal coumarins (hyuganin A, anomalin, pteryxin, isopteryxin, isoepoxypteryxin, praerosides II and IV, apiosylskimmin, (R)-peucedanol 7-O-beta-D-glucopyranoside), two acetylenic compounds [(-)-falcarinol and falcarindioll, and related compounds were examined for inhibitory activities on high concentration of K+ (High K+)- and dl-norepinephrine (NE)-induced contractions. The results indicate that the 3'- and 4'-acyl groups of khellactone-type coumarins are essential for the inhibitory activity on the contractions by High K+. Hyuganin A and anomalin showed inhibitory effects on High K+-induced contraction, but not on NE-induced contraction. Other active coumarins (pteryxin, isopteryxin, isoepoxypteryxin) and an acetylenic compound (falcarindiol) non-selectively inhibited both contractions by High K+ and NE.
Coumarins and antiplatelet aggregation constituents from Formosan Peucedanum japonicum.[Pubmed:8821432]
Phytochemistry. 1996 Feb;41(2):525-30.
Four new khellactone esters, (-)-trans-3'-acetyl-4'-senecioylkhellactone, (+-)-cis-3'-acetyl-4'-tigloylkhellactone, (+-)-cis-4-tigloylkhellactone, (+)-trans-4'-tigloylkhellactone, together with 14 known coumarins, isoimperatorin, psoralen, bergapten, xanthotoxol, cnidilin, (-)-selinidin, (-)-deltoin, (+)-pteryxin, (+)-peucedanocoumarin III, xanthotoxin, imperatorin, (+)-marmesin, (+)-oxypeucedanin hydrate, (+)-peucedanol and three chromones, eugenin, (-)-hamaudol, (+)-visamminol, have been isolated from the root of Formosan Peucedanum japonicum. The structures of the new compounds were elucidated by spectral data. The identities of (+)-trans-3'-tigloyl-4'-acetylkhellactone, formerly reported as a new compound, and (+)-cis-3'-angeloyl-4'-acetyl-khellactone, with the known (+)-peucedanocoumarin III and (+)-pteryxin, respectively, are discussed. Among the isolates, seven compounds, eugenin, (-)-selinidin, (+)-pteryxin, imperatorin, bergapten, cnidilin and (+)-visamminol, show strong antiplatelet aggregation activity in vitro.
Coumarin glycosides from Peucedanum japonicum.[Pubmed:7764824]
Phytochemistry. 1994 Mar 30;35(5):1339-41.
A new coumarin glycoside, peujaponiside [(R)-peucedanol 7-O-beta-D-apiofuranosyl-(1-->6)-beta-D-glucopyranoside], was isolated from the roots of Peucedanum japonicum, and its structure was elucidated on the basis of spectroscopic and chemical methods.


