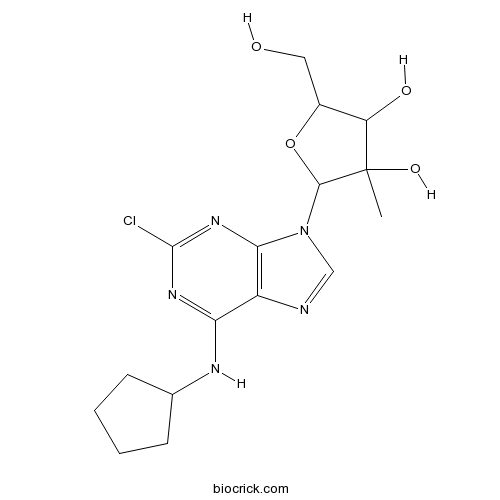
2'-MeCCPAVery selective and potent A1 receptor agonist CAS# 205171-12-6 |
- (24R)-MC 976
Catalog No.:BCC1289
CAS No.:112828-09-8
- (24S)-MC 976
Catalog No.:BCC1291
CAS No.:112849-14-6
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- 1alpha, 24, 25-Trihydroxy VD2
Catalog No.:BCC1298
CAS No.:457048-34-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 205171-12-6 | SDF | Download SDF |
| PubChem ID | 44292754 | Appearance | Powder |
| Formula | C16H22ClN5O4 | M.Wt | 383.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in DMSO | ||
| Chemical Name | 2-[2-chloro-6-(cyclopentylamino)purin-9-yl]-5-(hydroxymethyl)-3-methyloxolane-3,4-diol | ||
| SMILES | CC1(C(C(OC1N2C=NC3=C2N=C(N=C3NC4CCCC4)Cl)CO)O)O | ||
| Standard InChIKey | MMPAUXMIDJWGFO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H22ClN5O4/c1-16(25)11(24)9(6-23)26-14(16)22-7-18-10-12(19-8-4-2-3-5-8)20-15(17)21-13(10)22/h7-9,11,14,23-25H,2-6H2,1H3,(H,19,20,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and highly selective agonist at A1 adenosine receptors (Ki values are 3.3, 9580, 37600 and 1150 nM for human recombinant A1, A2A, A2B and A3 receptors respectively). Acts as a full agonist; inhibits forskolin-stimulated adenylyl cyclase activity in rat cortical membranes with an IC50 value of 13.1 nM. |

2'-MeCCPA Dilution Calculator

2'-MeCCPA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6053 mL | 13.0266 mL | 26.0532 mL | 52.1064 mL | 65.133 mL |
| 5 mM | 0.5211 mL | 2.6053 mL | 5.2106 mL | 10.4213 mL | 13.0266 mL |
| 10 mM | 0.2605 mL | 1.3027 mL | 2.6053 mL | 5.2106 mL | 6.5133 mL |
| 50 mM | 0.0521 mL | 0.2605 mL | 0.5211 mL | 1.0421 mL | 1.3027 mL |
| 100 mM | 0.0261 mL | 0.1303 mL | 0.2605 mL | 0.5211 mL | 0.6513 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Peusedanol
Catalog No.:BCC9119
CAS No.:20516-23-8
- Lansiumarin C
Catalog No.:BCN4895
CAS No.:205115-75-9
- Lansiumarin A
Catalog No.:BCN4894
CAS No.:205115-73-7
- Talatisamine
Catalog No.:BCN5403
CAS No.:20501-56-8
- 3,7,4'-Trihydroxy-5-methoxy-8-prenylflavanone
Catalog No.:BCN1503
CAS No.:204935-85-3
- Curzerenone
Catalog No.:BCN3009
CAS No.:20493-56-5
- Nardosinonediol
Catalog No.:BCN8118
CAS No.:20489-11-6
- Procumbide
Catalog No.:BCN3932
CAS No.:20486-27-5
- 8-O-Acetyltorilolone
Catalog No.:BCN7094
CAS No.:20482-21-7
- Fmoc-Lys(ivDde)-OH
Catalog No.:BCC3520
CAS No.:204777-78-6
- Olcegepant
Catalog No.:BCC1818
CAS No.:204697-65-4
- Epitaraxerol
Catalog No.:BCN4677
CAS No.:20460-33-7
- Isonardoperoxide
Catalog No.:BCN7628
CAS No.:205248-65-3
- DL-TBOA
Catalog No.:BCC5735
CAS No.:205309-81-5
- Amifostine
Catalog No.:BCC5232
CAS No.:20537-88-6
- Pinocembrin 7-O-(3'-galloyl-4',6'-(S)-hexahydroxydiphenoyl)-beta-D-glucose
Catalog No.:BCN6769
CAS No.:205370-59-8
- BU 224 hydrochloride
Catalog No.:BCC6765
CAS No.:205437-64-5
- Taxezopidine G
Catalog No.:BCN6947
CAS No.:205440-22-8
- Paederoside
Catalog No.:BCN3437
CAS No.:20547-45-9
- Rauvovertine B
Catalog No.:BCN7726
CAS No.:2055073-72-6
- Rauvovertine C
Catalog No.:BCN7727
CAS No.:2055073-74-8
- Rauvovertine A
Catalog No.:BCN7728
CAS No.:2055073-75-9
- Fmoc-D-Phe(3-Cl)-OH
Catalog No.:BCC3170
CAS No.:205526-23-4
- Fmoc-D-Phe(4-I)-OH
Catalog No.:BCC3262
CAS No.:205526-29-0
Synthesis, biological evaluation, and molecular modeling of ribose-modified adenosine analogues as adenosine receptor agonists.[Pubmed:15743197]
J Med Chem. 2005 Mar 10;48(5):1550-62.
A number of 3'-C-methyl analogues of selective adenosine receptor agonists such as CPA, CHA, CCPA, 2'-Me-CCPA, NECA, and IB-MECA was synthesized to further investigate the subdomain of the receptor that binds the ribose moiety of the ligands. Affinity data at A(1), A(2A), and A(3) receptors in bovine brain membranes showed that the 3'-C-modification in adenosine resulted in a decrease of the affinity at all three receptor subtypes. When this modification was combined with N(6)-substitution with groups that induce high potency and selectivity at A(1) receptor, the affinity and selectivity were increased. However, all 3'-C-methyl derivatives proved to be very less active than the corresponding 2'-C-methyl analogues. The most active compound was found to be 3'-Me-CPA which displayed a K(i) value of 0.35 microM at A(1) receptor and a selectivity for A(1) vs A(2A) and A(3) receptors higher than 28-fold. 2'-Me-CCPA was confirmed to be the most selective, high affinity agonist so far known also at human A(1) receptor with a K(i) value of 3.3 nM and 2903- and 341-fold selective vs human A(2A) and A(3) receptors, respectively. In functional assay, 3'-Me-CPA, 3'-Me-CCPA, and 2-Cl-3'-Me-IB-MECA inhibited forskolin-stimulated adenylyl cyclase activity with IC(50) values ranging from 0.3 to 4.9 microM, acting as full agonists. A rhodopsin-based model of the bovine A(1)AR was built to rationalize the higher affinity and selectivity of 2'-C-methyl derivatives of N(6)-substituted-adenosine compared to that of 3'-C-methyl analogues. In the docking exploration, it was found that 2'-Me-CCPA was able to form a number of interactions with several polar residues in the transmembrane helices TM-3, TM-6, and TM-7 of bA(1)AR which were not preserved in the molecular dynamics simulation of 3'-Me-CCPA/bA(1)AR complex.
2'-C-Methyl analogues of selective adenosine receptor agonists: synthesis and binding studies.[Pubmed:9572897]
J Med Chem. 1998 May 7;41(10):1708-15.
2'-C-Methyl analogues of selective adenosine receptor agonists such as (R)-PIA, CPA, CCPA, NECA, and IB-MECA were synthesized in order to further investigate the subdomain that binds the ribose moiety. Binding affinities of these new compounds at A1 and A2A receptors in bovine brain membranes and at A3 in rat testis membranes were determined and compared. It was found that the 2'-C-methyl modification resulted in a decrease of the affinity, particularly at A2A and A3 receptors. When such modification was combined with N6-substitutions with groups which induce high potency and selectivity at A1 receptors, the high affinity was retained and the selectivity was increased. Thus, 2-chloro-2'-C-methyl-N6-cyclopentyladenosine (2'-Me-CCPA), which displayed a Ki value of 1.8 nM at A1 receptors, was selective for A1 vs A2A and A3 receptors by 2166- and 2777-fold, respectively, resulting in one of the most potent and A1-selective agonists so far known. In functional assay, this compound inhibited forskolin-stimulated adenylyl cyclase activity with an IC50 value of 13.1 nM, acting as a full agonist.


