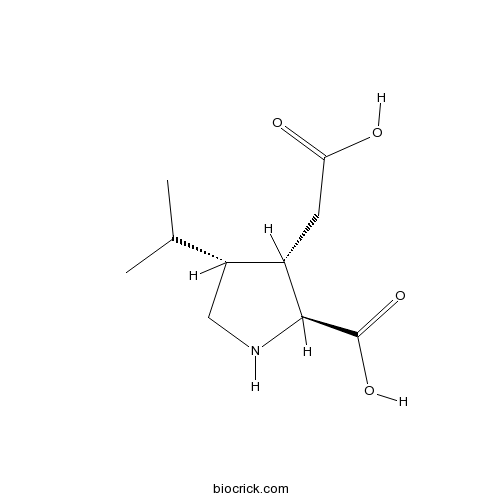
Dihydrokainic acidEAAT2 (GLT-1)-selective non-transportable inhibitor of L-glutamate and L-aspartate uptake CAS# 52497-36-6 |
- MLN9708
Catalog No.:BCC2091
CAS No.:1201902-80-8
- MG-115
Catalog No.:BCC1237
CAS No.:133407-86-0
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- CEP-18770
Catalog No.:BCC2093
CAS No.:847499-27-8
- Carfilzomib (PR-171)
Catalog No.:BCC1145
CAS No.:868540-17-4
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 52497-36-6 | SDF | Download SDF |
| PubChem ID | 107883 | Appearance | Powder |
| Formula | C10H17NO4 | M.Wt | 215.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
| Chemical Name | (2S,3S,4R)-3-(carboxymethyl)-4-propan-2-ylpyrrolidine-2-carboxylic acid | ||
| SMILES | CC(C)C1CNC(C1CC(=O)O)C(=O)O | ||
| Standard InChIKey | JQPDCKOQOOQUSC-OOZYFLPDSA-N | ||
| Standard InChI | InChI=1S/C10H17NO4/c1-5(2)7-4-11-9(10(14)15)6(7)3-8(12)13/h5-7,9,11H,3-4H2,1-2H3,(H,12,13)(H,14,15)/t6-,7+,9-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | EAAT2(GLT1)-selective non-transportable inhibitor of L-glutamate and L-aspartate uptake (Ki = 23 μM). 130-fold selective over EAAT1 and EAAT3 (Ki > 3 mM). Also available as part of the Excitatory Amino Acid Transporter Inhibitor. |

Dihydrokainic acid Dilution Calculator

Dihydrokainic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.6458 mL | 23.2288 mL | 46.4576 mL | 92.9152 mL | 116.144 mL |
| 5 mM | 0.9292 mL | 4.6458 mL | 9.2915 mL | 18.583 mL | 23.2288 mL |
| 10 mM | 0.4646 mL | 2.3229 mL | 4.6458 mL | 9.2915 mL | 11.6144 mL |
| 50 mM | 0.0929 mL | 0.4646 mL | 0.9292 mL | 1.8583 mL | 2.3229 mL |
| 100 mM | 0.0465 mL | 0.2323 mL | 0.4646 mL | 0.9292 mL | 1.1614 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PSB 36
Catalog No.:BCC7240
CAS No.:524944-72-7
- Peonidin-3-O-arabinoside chloride
Catalog No.:BCN3029
CAS No.:524943-91-7
- D-Tryptophanol
Catalog No.:BCC2699
CAS No.:52485-52-6
- 5-(Z-heptadec-8-enyl) resorcinol
Catalog No.:BCN5674
CAS No.:52483-19-9
- Isokurarinone
Catalog No.:BCN2890
CAS No.:52483-02-0
- Vandetanib hydrochloride
Catalog No.:BCC2028
CAS No.:524722-52-9
- ERB 041
Catalog No.:BCC7903
CAS No.:524684-52-4
- 1-Cinnamoylpyrrolidine
Catalog No.:BCN4086
CAS No.:52438-21-8
- Isobutylshikonin
Catalog No.:BCN3005
CAS No.:52438-12-7
- Oxeladin Citrate
Catalog No.:BCC3831
CAS No.:52432-72-1
- Cochlearine
Catalog No.:BCN1929
CAS No.:52418-07-2
- Boc-D-Met-OH
Catalog No.:BCC3426
CAS No.:5241-66-7
- Fraxidin
Catalog No.:BCN5675
CAS No.:525-21-3
- Harmalol
Catalog No.:BCC8183
CAS No.:525-57-5
- Kinetin
Catalog No.:BCC1679
CAS No.:525-79-1
- Flavone
Catalog No.:BCN8477
CAS No.:525-82-6
- Quercetin 3-O-robinobioside
Catalog No.:BCN5676
CAS No.:52525-35-6
- 5,7-Dihydroxychromone 7-rutinoside
Catalog No.:BCN3333
CAS No.:52538-46-2
- Pranoprofen
Catalog No.:BCC4828
CAS No.:52549-17-4
- Ingenol 3-palmitate
Catalog No.:BCN7686
CAS No.:52557-26-3
- bPiDDB
Catalog No.:BCC7606
CAS No.:525596-66-1
- Glycozolinine
Catalog No.:BCN5677
CAS No.:5257-08-9
- Acm-thiopropionic acid
Catalog No.:BCC2838
CAS No.:52574-08-0
- Phellamurin
Catalog No.:BCN5678
CAS No.:52589-11-4
Effects of dihydrokainic acid on extracellular amino acids and neuronal excitability in the in vivo rat hippocampus.[Pubmed:2882438]
Neuropharmacology. 1987 Jan;26(1):1-8.
The effect of inhibition of the high-affinity uptake of glutamate on the extracellular concentration of amino acids and on neuronal excitability was studied in vivo in the hippocampus of the rat. The dentate gyrus or CA1 field were perfused through a dialytrode with Krebs-Ringer-bicarbonate or Dihydrokainic acid solutions. The spontaneous electrical activity and evoked field potentials were recorded concomitantly at dendritic or somatic levels. The results showed that with Dihydrokainic acid: the extracellular concentrations of both glutamate and taurine were markedly increased in both areas of the hippocampus, the response of taurine being greater in CA1, while other amino acids were unaffected; in the dentate gyrus, the field excitatory postsynaptic potential was decreased while the population spikes were augmented, indicating an increased excitability of the neuronal population. In CA1, both the excitatory postsynaptic potential and spikes were reduced in amplitude. These results indicate that changes in the extracellular concentration of endogenous glutamate influences excitability of the tissue and that inhibition of the uptake processes for putative amino acid neurotransmitters increases the postsynaptic action of synaptically-released endogenous amino acids.
On the epileptogenic effects of kainic acid and dihydrokainic acid in the dentate gyrus of the rat.[Pubmed:3419538]
Neuropharmacology. 1988 Apr;27(4):375-81.
The in vivo effects of the acidic amino receptor agonist, kainic acid and the inhibitors of the uptake of glutamate, Dihydrokainic acid and threo-3-hydroxyaspartate, on spontaneous activity and perforant path evoked field potentials were examined in the dentate gyrus of the rat. The effect of these compounds on extracellular levels of endogenous amino acids in the hippocampus was assessed simultaneously using in vivo microdialysis. Kainic acid (10-100 microM) and Dihydrokainic acid (1-10 mM) both evoked epileptiform activity and an apparent loss of recurrent inhibition (as assessed using the paired-pulse technique). Extracellular increases in taurine, alanine and phosphoethanolamine were noted following administration of kainate (100 microM) and dihydrokainate (1-10 mM). An increase in extracellular glutamate and aspartate was also noted in rats treated with dihydrokainate (100 microM-10 mM). In contrast, threo-3-hydroxyaspartate did not induce epileptiform activity, suggesting that the epileptogenic effects of dihydrokainate and kainate are not mediated by inhibition of uptake. The effect of the N-methyl-D-aspartate receptor antagonist, D-2-amino-5-phosphonovalerate on these responses was studied. This compound attenuated the epileptiform activity and reversed the apparent loss of recurrent inhibition in response to both kainic acid and Dihydrokainic acid. These data suggest that activation of N-methyl-D-aspartate receptors underlies the epileptogenic effects of both compounds, and the possible mechanisms which might be involved in this response are discussed.
Kainic acid, AMPA, and dihydrokainic acid effect on uptake and efflux of D-[3H] aspartic acid in cerebellar slices.[Pubmed:8953569]
Neurochem Res. 1996 Dec;21(12):1527-33.
In this study we show that the glutamate ionotropic agonist kainate (KA) stimulates the efflux of preloaded D-[3H]aspartate (D-[3H]Asp) and inhibits the uptake of this amino acid in cerebellar slices. The effect of this agonist on the efflux of D-[3H]Asp is sensitive to (i) 6-nitro-7-sulphamoylbenzo(f)quinoxaline-2-3-dione (NBQX), indicating the involvement of KA/(RS)-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and is (ii) partially tetrodotoxin (TTX)-sensitive, indicating that pre-(TTX-insensitive) and post-synaptic (TTX-sensitive) KA/AMPA receptors are involved. In contrast, the effect on uptake is NBQX- and TTX-insensitive indicating a direct interaction with glutamate transporters. AMPA inhibited D-[3H]Asp uptake and had no effect on D-[3H]Asp efflux. In the same system, the uptake but not the efflux of D-[3H]Asp was affected by dihydrokainate (DHK). The DHK-induced uptake inhibition occurred in the presence of TTX. NBQX inhibited DHK-induced effect at 5 mM but not at 1 mM DHK concentrations.
Blood-brain barrier permeability and brain uptake mechanism of kainic acid and dihydrokainic acid.[Pubmed:25488153]
Neurochem Res. 2015 Mar;40(3):542-9.
The glutamatergic neurotransmitter system is involved in important neurophysiological processes and thus constitutes a promising target for the treatment of neurological diseases. The two ionotropic glutamate receptor agonists kainic acid (KA) and Dihydrokainic acid (DHK) have been used as research tools in various in vivo central nervous system disease models in rodents, as well as being templates in the design of novel ligands affecting the glutamatergic system. Both molecules are highly polar but yet capable of crossing the blood-brain barrier (BBB). We used an in situ rat brain perfusion technique to determine the brain uptake mechanism and permeability across the BBB. To determine KA and DHK concentrations in the rat brain, simple and rapid sample preparation and liquid chromatography mass spectrometer methods were developed. According to our results the BBB permeability of KA and DHK is low, 0.25 x 10(-6) and 0.28 x 10(-6) cm/s for KA and DHK, respectively. In addition, the brain uptake is mediated by passive diffusion, and not by active transport. Furthermore, the non-specific plasma and brain protein binding of KA and DHK was determined to be low, which means that the unbound drug volume of distribution in brain is also low. Therefore, even though the total KA and DHK concentrations in the brain are low after systemic dosing, the concentrations in the vicinity of the glutamate receptors are sufficient for their activation and thus the observed efficacy.
Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex.[Pubmed:7521911]
J Neurosci. 1994 Sep;14(9):5559-69.
Reuptake plays an important role in regulating synaptic and extracellular concentrations of glutamate. Three glutamate transporters expressed in human motor cortex, termed EAAT1, EAAT2, and EAAT3 (for excitatory amino acid transporter), have been characterized by their molecular cloning and functional expression. Each EAAT subtype mRNA was found in all human brain regions analyzed. The most prominent regional variation in message content was in cerebellum where EAAT1 expression predominated. EAAT1 and EAAT3 mRNAs were also expressed in various non-nervous tissues, whereas expression of EAAT2 was largely restricted to brain. The kinetic parameters and pharmacological characteristics of transport mediated by each EAAT subtype were determined in transfected mammalian cells by radio-label uptake and in microinjected oocytes by voltage-clamp measurements. The affinities of the EAAT subtypes for L-glutamate were similar, with Km determinations varying from 48 to 97 microM in the mammalian cell assay and from 18 to 28 microM in oocytes. Glutamate uptake inhibitors were used to compare the pharmacologies of the EAAT subtypes. The EAAT2 subtype was distinguishable from the EAAT1/EAAT3 subtypes by the potency of several inhibitors, but most notably by sensitivity to kainic acid (KA) and Dihydrokainic acid (DHK). KA and DHK potently inhibited EAAT2 transport, but did not significantly affect transport by EAAT1/EAAT3. Using voltage-clamp measurements, most inhibitors were found to be substrates that elicited transport currents. In contrast, KA and DHK did not evoke currents and they were found to block EAAT2-mediated transport competitively. This selective interaction with the EAAT2 subtype could be a significant factor in KA neurotoxicity. These studies provide a foundation for understanding the role of glutamate transporters in human excitatory neurotransmission and in neuropathology.
The neuronal and epithelial human high affinity glutamate transporter. Insights into structure and mechanism of transport.[Pubmed:7914198]
J Biol Chem. 1994 Aug 12;269(32):20599-606.
High affinity transport of glutamate across plasma membranes of brain neurons and epithelial is mediated by a Na(+)- and K(+)-coupled electrogenic transporter. Here we report the primary structure and functional characterization of the human high affinity glutamate transporter (HEAAC1). A unique characteristic of HEAAC1-mediated transport is that the affinity for glutamate and the maximal transport rate are strongly dependent on membrane potential. Our data provide new insights into individual steps of high affinity glutamate transport and show that the transport mechanism is distinct from that of the gamma-aminobutyric acid transporter GAT-1 and the Na+/glucose transporter SGLT1. Under voltage clamp condition, HEAAC1 mediated large substrate-evoked inward currents (up to 1 microA). The substrate specificity, stereospecificity, the Km value (30 +/- 3 microM at -60 mV) of the L-glutamate-evoked current, and Northern analysis all agree with previously reported characteristics of high affinity glutamate transport in brain. In contrast to SGLT1 and GAT-1, voltage jump studies of HEAAC1 yielded only minor relaxation currents. Classic inhibitors of brain glutamate uptake such as DL-threo-beta-hydroxyaspartate, L-trans-pyrrolidine 2,4,-dicarboxylic acid (PDC), and dihydrokainate were found to be either transport substrates or to have no significant effect on glutamate transport. We also found that the maximal transport rate for PDC was markedly reduced compared to that for L-glutamate. We propose that PDC most likely reduces the turnover rate of the transporter. A search of the sequence data bases revealed weak homology of HEAAC1 to the H(+)-coupled vesicular monoamine transporter, suggesting an evolutionary link between plasma membrane and vesicular transporters.


