Congo RedCAS# 573-58-0 |
- Brefeldin A
Catalog No.:BCC4387
CAS No.:20350-15-6
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- Bafilomycin A1
Catalog No.:BCC3914
CAS No.:88899-55-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 573-58-0 | SDF | Download SDF |
| PubChem ID | 11313 | Appearance | Powder |
| Formula | C32H22N6Na2O6S2 | M.Wt | 696.66 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water and to 10 mM in DMSO | ||
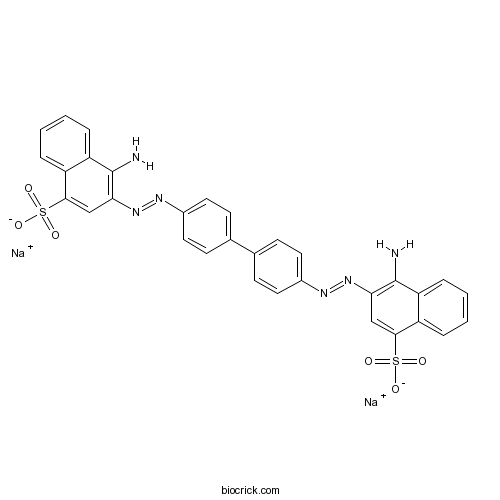
| Chemical Name | disodium;4-amino-3-[[4-[4-[(1-amino-4-sulfonatonaphthalen-2-yl)diazenyl]phenyl]phenyl]diazenyl]naphthalene-1-sulfonate | ||
| SMILES | C1=CC=C2C(=C1)C(=CC(=C2N)N=NC3=CC=C(C=C3)C4=CC=C(C=C4)N=NC5=C(C6=CC=CC=C6C(=C5)S(=O)(=O)[O-])N)S(=O)(=O)[O-].[Na+].[Na+] | ||
| Standard InChIKey | IQFVPQOLBLOTPF-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/C32H24N6O6S2.2Na/c33-31-25-7-3-1-5-23(25)29(45(39,40)41)17-27(31)37-35-21-13-9-19(10-14-21)20-11-15-22(16-12-20)36-38-28-18-30(46(42,43)44)24-6-2-4-8-26(24)32(28)34;;/h1-18H,33-34H2,(H,39,40,41)(H,42,43,44);;/q;2*+1/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | VGlut inhibitor; decreases synaptic transmission in the dentate gyrus. Also an amyloid fibril-binding dye; inhibits Fib-beta A neurotoxicity by inhibiting fibril formation or by binding to preformed fibrils. Also inhibits the pancreatic islet cell toxicity of diabetes-associated amylin. Excitation wavelength is 497 nm. |

Congo Red Dilution Calculator

Congo Red Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4354 mL | 7.1771 mL | 14.3542 mL | 28.7084 mL | 35.8855 mL |
| 5 mM | 0.2871 mL | 1.4354 mL | 2.8708 mL | 5.7417 mL | 7.1771 mL |
| 10 mM | 0.1435 mL | 0.7177 mL | 1.4354 mL | 2.8708 mL | 3.5886 mL |
| 50 mM | 0.0287 mL | 0.1435 mL | 0.2871 mL | 0.5742 mL | 0.7177 mL |
| 100 mM | 0.0144 mL | 0.0718 mL | 0.1435 mL | 0.2871 mL | 0.3589 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Congo red is an azo dye. Congo red (CR) binding been used as a diagnostic test for the presence of amyloid in tissue sections.
In Vitro:Congo red histochemical stain may serve as a simple screening tool for investigating if the aggregates in mutant cells have misfolded β-pleated sheet secondary structures. Congo red histochemical dye has the ability to bind specifically to crossed β-pleated sheet structures. Wild-type HSPB1 should maintain protein homeostasis by binding proteins in non-native conformations, thereby preventing substrate aggregation. The T139M mutant, however, fails in this function and results in an accumulation of misfolded proteins, which are targeted by Congo red for intercalating between the β-pleated sheet structures. Congo red histochemical stain may serve as a simple tool to investigate if the aggregates in mutant cells have misfolded β-pleated sheet secondary structures[1].
References:
[1]. Amornvit J, et al. A novel p.T139M mutation in HSPB1 highlighting the phenotypic spectrum in a family. Brain Behav. 2017 Jul 21;7(8):e00774.
- Liriodendrin
Catalog No.:BCN5774
CAS No.:573-44-4
- Boehmenan
Catalog No.:BCN5773
CAS No.:57296-22-7
- Ridaforolimus (Deforolimus, MK-8669)
Catalog No.:BCC4605
CAS No.:572924-54-0
- Boc-D-Phe(4-F)-OH
Catalog No.:BCC3218
CAS No.:57292-45-2
- Boc-D-Phe(4-Cl)-OH
Catalog No.:BCC3176
CAS No.:57292-44-1
- Calmidazolium chloride
Catalog No.:BCC7410
CAS No.:57265-65-3
- Setiptiline
Catalog No.:BCC1945
CAS No.:57262-94-9
- Salsolinol-1-carboxylic acid
Catalog No.:BCC6731
CAS No.:57256-34-5
- 7-Ethoxyresorufin
Catalog No.:BCC6476
CAS No.:5725-91-7
- Methoxyresorufin
Catalog No.:BCC6296
CAS No.:5725-89-3
- Pamidronate Disodium
Catalog No.:BCC1193
CAS No.:57248-88-1
- H-Phe(3-CN)-OH
Catalog No.:BCC3182
CAS No.:57213-48-6
- Tacalcitol
Catalog No.:BCC1975
CAS No.:57333-96-7
- Dihydroepistephamiersine 6-acetate
Catalog No.:BCN5775
CAS No.:57361-74-7
- Bombinakinin-GAP
Catalog No.:BCC5903
CAS No.:573671-91-7
- Isochlorogenic acid C
Catalog No.:BCN2498
CAS No.:57378-72-0
- Irsogladine
Catalog No.:BCC4562
CAS No.:57381-26-7
- Oroxin A
Catalog No.:BCN1202
CAS No.:57396-78-8
- Benzoin ethyl ether
Catalog No.:BCC8855
CAS No.:574-09-4
- Isoflavone
Catalog No.:BCN8508
CAS No.:574-12-9
- Fraxetin
Catalog No.:BCN5903
CAS No.:574-84-5
- Fexaramine
Catalog No.:BCC7412
CAS No.:574013-66-4
- 8-O-Acetylshanzhiside methyl ester
Catalog No.:BCN5776
CAS No.:57420-46-9
- (2S)-2alpha-(1,3-Benzodioxol-5-yl)-3,5-dihydro-5alpha-methoxy-3beta-methyl-5-allyl-2H-benzofuran-6-one
Catalog No.:BCN6606
CAS No.:57430-03-2
Combined thioflavin T-Congo red fluorescence assay for amyloid fibril detection.[Pubmed:28355156]
Methods Appl Fluoresc. 2016 Sep 6;4(3):034010.
Fluorescence represents one of the most powerful tools for the detection and structural characterization of the pathogenic protein aggregates, amyloid fibrils. The traditional approaches to the identification and quantification of amyloid fibrils are based on monitoring the fluorescence changes of the benzothiazole dye thioflavin T (ThT) and absorbance changes of the azo dye Congo Red (CR). In routine screening it is usually sufficient to perform only the ThT and CR assays, but both of them, when used separately, could give false results. Moreover, fibrillization kinetics can be measured only by ThT fluorescence, while the characteristic absorption spectra and birefringence of CR represent more rigid criteria for the presence of amyloid fibrils. Therefore, it seemed reasonable to use both these dyes simultaneously, combining the advantages of each technique. To this end, we undertook a detailed analysis of the fluorescence spectral behavior of these unique amyloid tracers upon their binding to amyloid fibrils from lysozyme, insulin and an N-terminal fragment of apolipoprotein A-I with Iowa mutation. The fluorescence measurements revealed several criteria for distinguishing between fibrillar and monomeric protein states: (i) a common drastic increase in ThT fluorescence intensity; (ii) a sharp decrease in ThT fluorescence upon addition of CR; (iii) an appearance of the maximum at 535-540 nm in the CR excitation spectra; (iv) increase in CR fluorescence intensity at 610 nm. Based on these findings we designed a novel combined ThT-CR fluorescence assay for amyloid identification. Such an approach not only strengthens the reliability of the ThT assay, but also provides new opportunities for structural characterization of amyloid fibrils.
Equilibrium isotherms, kinetics, and thermodynamics studies for congo red adsorption using calcium alginate beads impregnated with nano-goethite.[Pubmed:28349874]
Ecotoxicol Environ Saf. 2017 Jul;141:226-234.
The present study is concerned with the batch adsorption of Congo Red (CR) from an aqueous solution using calcium alginate beads impregnated with nano-goethite (CABI nano-goethite) as an adsorbent. The optimum conditions for CR removal were determined by studying operational variables viz. pH, adsorbent dose, contact time, initial dye ion concentration and temperature. The CABI nano-goethite was characterized by Fourier transform infrared spectroscopy (FTIR), X- ray diffraction (XRD) and Scanning electron microscopy/energy dispersive spectroscopy (SEM/EDS) analysis. The CR sorption data onto CABI nano-goethite were described using Langmuir, Freundlich, Dubinin-Radushkevich and Temkin isotherm models. The results show that the best fit was achieved with the Langmuir isotherm model. The maximum adsorption capacity (181.1mg/g) of CR was occurred at pH 3.0. Kinetic studies showed that the adsorption followed a pseudo-second-order model. Desorption experiments were carried out to explore the feasibility of regenerating the adsorbent and the adsorbed CR from CABI nano-goethite. The best desorbing agent was 0.1M NaOH with an efficiency of 94% recovery. The thermodynamic parameters DeltaG degrees , DeltaH degrees , and DeltaS degrees for the CR adsorption were determined by using adsorption capacities at five different temperatures (293, 303, 313, 323 and 303K). Results show that the adsorption process was endothermic and favoured at high temperature.
Anion selective pTSA doped polyaniline@graphene oxide-multiwalled carbon nanotube composite for Cr(VI) and Congo red adsorption.[Pubmed:28242347]
J Colloid Interface Sci. 2017 Jun 15;496:407-415.
Multiwalled carbon nanotube (CNT)-graphene oxide (GO) composite was combined with polyaniline (Pani) using an oxidative polymerisation technique. The resulting Pani@GO-CNT was later doped with para toluene sulphonic acid (pTSA) to generate additional functionality. The functional groups exposed on the GO, Pani and pTSA were expected to impart a high degree of functionality to the pTSA-Pani@GO-CNT composite system. The composite was characterised by scanning electron microscopy, transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy. The characterisation results revealed the characteristics of Pani, GO, CNT, and pTSA, and suggested the successful formation of the pTSA-Pani@GO-CNT composite system. The composite was utilised successfully for the adsorptive removal of Cr(IV) and Congo Red (CR) dye and the adsorption of both pollutants was found to be strongly dependent on the solution pH, adsorbate concentration, contact time, and reaction temperature. The maximum adsorption of Cr(IV) and CR was observed in an acidic medium at 30 degrees C. The kinetics for Cr(IV) and CR adsorption was studied using pseudo-first order, pseudo-second order, and intraparticle diffusion models. The adsorption equilibrium data were also fitted to the Langmuir and Freundlich isotherm models. The thermodynamic results showed that the adsorption process was exothermic in nature. The present study provides a new methodology for the preparation of a highly functionalised Pani-based nanocomposite system and its potential applications to the adsorptive removal of a multicomponent pollutant system from an aqueous solution.
Full Genome Characterization of a New Simian Immune Deficiency Virus Lineage in a Naturally Infected Cercopithecus ascanius whitesidei in the Democratic Republic of Congo Reveals High Genetic Diversity Among Red-Tailed Monkeys in Central and Eastern Africa.[Pubmed:28383997]
AIDS Res Hum Retroviruses. 2017 Jul;33(7):735-739.
Our knowledge on simian immune deficiency virus (SIV) diversity and evolution in the different nonhuman primate species is still incomplete. In this study, we report the full genome characterization of a new SIV from a red-tailed monkey (2013DRC-I8), from the Cercopithecus ascanius whitesidei subspecies, in the Democratic Republic of Congo (DRC). The new full-length genome is 9,926 bp long, and the genomic structure is similar to that of other SIVs with the absence of vpx and vpu genes. The new SIVasc-13DRC-I8 strain fell within the Cercopithecus specific SIV lineage. SIVasc-13DRC-I8 and previously reported SIVrtg from the C.a. schmidti subspecies in Uganda did not form a separate species-specific SIV lineage. These observations provide additional evidence for high genetic diversity and the complex evolution of SIVs in the Cercopithecus genus. More studies on a large number of monkeys from a wider geographic area are needed to understand SIV evolution.
Modulation of hippocampal synaptic transmission by the kynurenine pathway member xanthurenic acid and other VGLUT inhibitors.[Pubmed:23303071]
Neuropsychopharmacology. 2013 May;38(6):1060-7.
Xanthurenic acid (XA), an endogenous kynurenine, is a known vesicular glutamate transport (VGLUT) inhibitor and has also been proposed as an mGlu2/3 receptor agonist. Changes in these systems have been implicated in the pathophysiology of schizophrenia and other psychiatric disorders; however, little is known of how XA affects synaptic transmission. We therefore investigated the effects of XA on synaptic transmission at two hippocampal glutamatergic pathways and evaluated the ability of XA to bind to mGlu2/3 receptors. Field excitatory postsynaptic potentials (fEPSPs) were recorded from either the dentate gyrus (DG) or CA1 region of mouse hippocampal slices in vitro. Addition of XA to the bathing medium (1-10 mM) resulted in a dose-related reduction of fEPSP amplitudes (up to 52% reduction) in both hippocampal regions. In the DG, the VGLUT inhibitors Congo Red and Rose Bengal, and the mGlu2/3 agonist LY354740, also reduced fEPSPs (up to 80% reduction). The mGlu2/3 antagonist LY341495 reversed the LY354740 effect, but not the XA effect. LY354740, but not XA, also reduced DG paired-pulse depression. XA had no effect on specific binding of 1 nM [(3)H]LY341495 to membranes with human mGlu2 receptors. We conclude that XA can modulate synaptic transmission via a mechanism that may involve VGLUT inhibition rather than activation of mGlu2/3 receptors. This could be important in the pathophysiology of nervous system disorders including schizophrenia and might represent a target for developing novel pharmacological therapies.
Effect of VGLUT inhibitors on glutamatergic synaptic transmission in the rodent hippocampus and prefrontal cortex.[Pubmed:24121008]
Neurochem Int. 2014 Jul;73:159-65.
Vesicular glutamate transporters (VGLUTs) are known to be important in the uptake of glutamate into vesicles in the presynaptic terminal; thereby playing a role in synaptic function. VGLUT dysfunction has also been suggested in neurological and psychiatric disorders such as epilepsy and schizophrenia. A number of compounds have been identified as VGLUT inhibitors; however, little is known as to how these compounds affect synaptic transmission. We therefore investigated the effects of structurally unrelated VGLUT inhibitors on synaptic transmission in the rodent hippocampus and prefrontal cortex. In the CA1 and dentate gyrus regions of the in vitro slice preparation of mouse hippocampus, AMPA receptor-mediated field excitatory postsynaptic potentials (fEPSPs) were evoked in response to Schaffer collateral/commissural pathway stimulation. Application of the VGLUT inhibitors Rose Bengal (RB), Congo Red (CR) or Chicago Sky Blue 6B (CB) resulted in a concentration-related reduction of fEPSP amplitudes. RB (30muM) or CB (300muM) also depressed NMDA receptor-mediated responses in the CA1 region. The naturally occurring kynurenine Xanthurenic Acid (XA) is reported to be a VGLUT inhibitor. We found XA attenuated both AMPA and NMDA receptor-mediated synaptic transmission. The potency order of the VGLUT inhibitors was consistent with literature Ki values for VGLUT inhibition. Impaired glutamatergic neurotransmission is believed to contribute to schizophrenia, and VGLUTs have also been implicated in this disease. We therefore investigated the effect of VGLUT inhibition in the prefrontal cortex. Application of the VGLUT inhibitors RB or CB resulted in a concentration-dependent reduction in the amplitude of glutamate receptor-mediated fEPSPs recorded in layer V/VI in response to stimulation in the forceps minor. We conclude that VGLUT inhibitors can modulate glutamatergic synaptic transmission in the PFC and hippocampus. This could be important in the pathophysiology of nervous system disorders and might represent a target for developing novel pharmacological therapies.
Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red.[Pubmed:7991613]
Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12243-7.
beta-Amyloid (beta A) is normally produced as a nontoxic soluble peptide. In Alzheimer disease, beta A aggregates and accumulates in the brain as inert diffuse plaques or compact plaques associated with neurodegenerative changes. To determine the relationship of neurotoxicity to the physical state of beta A, we created (i) nonamyloidogenic amorphous aggregates of beta A [amorphous beta A (Am-beta A)] analogous to diffuse plaques and (ii) amyloidogenic fibrils of beta A [fibrillar beta A (Fib-beta A)] analogous to compact plaques. In primary rat hippocampal culture, Fib-beta A was neurotoxic, whereas Am-beta A was not toxic. Fib-beta A caused significant loss of synapses in viable neurons, while Am-beta A had no effect on synapse number. The amyloid fibril-binding dye Congo Red inhibited Fib-beta A neurotoxicity by inhibiting fibril formation or by binding to preformed fibrils. Congo Red also inhibited the pancreatic islet cell toxicity of diabetes-associated amylin, another type of amyloid fibril. These results indicate that beta A neurotoxicity requires fibril formation. These findings and our previous demonstration that amylin fibrils are toxic suggest that a common cytopathic effect of amyloid fibrils may contribute to the pathogenesis of Alzheimer disease and other amyloidoses.


