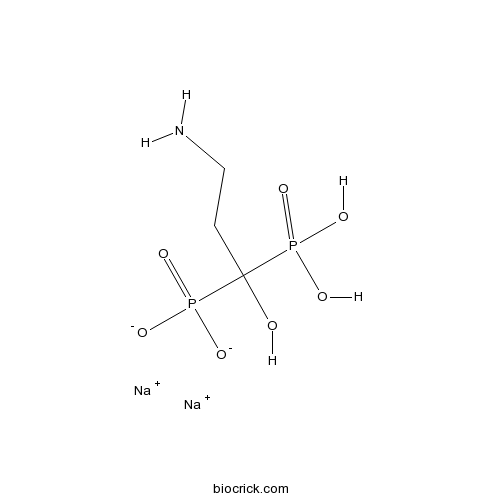
Pamidronate DisodiumBisphosphonate antiresorptive agent & bone resorption inhibitor CAS# 57248-88-1 |
- GDC-0068 (RG7440)
Catalog No.:BCC1271
CAS No.:1001264-89-6
- MK-2206 dihydrochloride
Catalog No.:BCC1274
CAS No.:1032350-13-2
- AZD5363
Catalog No.:BCC1073
CAS No.:1143532-39-1
- A-443654
Catalog No.:BCC1321
CAS No.:552325-16-3
- AKT inhibitor VIII
Catalog No.:BCC1334
CAS No.:612847-09-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 57248-88-1 | SDF | Download SDF |
| PubChem ID | 44181920 | Appearance | Powder |
| Formula | C3H9NNa2O7P2 | M.Wt | 279.03 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CGP 23339A | ||
| Solubility | Soluble to 56 mg/mL (200.69 mM) in Water | ||
| Chemical Name | disodium;(3-amino-1-hydroxy-1-phosphonatopropyl)phosphonic acid | ||
| SMILES | C(CN)C(O)(P(=O)(O)O)P(=O)([O-])[O-].[Na+].[Na+] | ||
| Standard InChIKey | CEYUIFJWVHOCPP-UHFFFAOYSA-L | ||
| Standard InChI | InChI=1S/C3H11NO7P2.2Na/c4-2-1-3(5,12(6,7)8)13(9,10)11;;/h5H,1-2,4H2,(H2,6,7,8)(H2,9,10,11);;/q;2*+1/p-2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pamidronate disodium, a bisphosphonate drug, can help to strengthen bones.
Target: Others
Pamidronate belongs to the family of medications known as bisphosphonates. It is used to treat hypercalcemia (high blood calcium) by people who have cancer. Pamidronate is also used to treat cancer that has spread to bones (bone metastases) due to different types of tumours and multiple myeloma (cancer of the bone marrow). Pamidronate is also used to treat the symptoms of Paget's disease of bone. References: | |||||

Pamidronate Disodium Dilution Calculator

Pamidronate Disodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5838 mL | 17.9192 mL | 35.8384 mL | 71.6769 mL | 89.5961 mL |
| 5 mM | 0.7168 mL | 3.5838 mL | 7.1677 mL | 14.3354 mL | 17.9192 mL |
| 10 mM | 0.3584 mL | 1.7919 mL | 3.5838 mL | 7.1677 mL | 8.9596 mL |
| 50 mM | 0.0717 mL | 0.3584 mL | 0.7168 mL | 1.4335 mL | 1.7919 mL |
| 100 mM | 0.0358 mL | 0.1792 mL | 0.3584 mL | 0.7168 mL | 0.896 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A bisphosphonate antiresorptive agent and a bone resorption inhibitor.
- H-Phe(3-CN)-OH
Catalog No.:BCC3182
CAS No.:57213-48-6
- Testosterone decanoate
Catalog No.:BCC9168
CAS No.:5721-91-5
- Ayanin
Catalog No.:BCN4056
CAS No.:572-32-7
- Engeletin
Catalog No.:BCN5772
CAS No.:572-31-6
- Avicularin
Catalog No.:BCN5771
CAS No.:572-30-5
- Neosenkirkine
Catalog No.:BCN2138
CAS No.:57194-70-4
- Paxilline
Catalog No.:BCC7235
CAS No.:57186-25-1
- Naftopidil DiHCl
Catalog No.:BCC4355
CAS No.:57149-08-3
- Naftopidil
Catalog No.:BCC4352
CAS No.:57149-07-2
- 16,17-Dihydroapovincamine
Catalog No.:BCN8049
CAS No.:57130-30-0
- Erastin
Catalog No.:BCC4497
CAS No.:571203-78-6
- Laropiprant
Catalog No.:BCC1688
CAS No.:571170-77-9
- Methoxyresorufin
Catalog No.:BCC6296
CAS No.:5725-89-3
- 7-Ethoxyresorufin
Catalog No.:BCC6476
CAS No.:5725-91-7
- Salsolinol-1-carboxylic acid
Catalog No.:BCC6731
CAS No.:57256-34-5
- Setiptiline
Catalog No.:BCC1945
CAS No.:57262-94-9
- Calmidazolium chloride
Catalog No.:BCC7410
CAS No.:57265-65-3
- Boc-D-Phe(4-Cl)-OH
Catalog No.:BCC3176
CAS No.:57292-44-1
- Boc-D-Phe(4-F)-OH
Catalog No.:BCC3218
CAS No.:57292-45-2
- Ridaforolimus (Deforolimus, MK-8669)
Catalog No.:BCC4605
CAS No.:572924-54-0
- Boehmenan
Catalog No.:BCN5773
CAS No.:57296-22-7
- Liriodendrin
Catalog No.:BCN5774
CAS No.:573-44-4
- Congo Red
Catalog No.:BCC8023
CAS No.:573-58-0
- Tacalcitol
Catalog No.:BCC1975
CAS No.:57333-96-7
Hypertrophic osteoarthropathy presenting as unilateral cellulitis with successful treatment using pamidronate disodium.[Pubmed:23050033]
J Clin Aesthet Dermatol. 2012 Sep;5(9):37-46.
Hypertrophic pulmonary osteoarthropathy is a paraneoplastic syndrome seen in patients with lung cancer. This condition is characterized by the presence of digital clubbing, periosteal thickening, synovial thickening, and severe pain of the affected joints. Other syndromes exhibiting clubbing may or may not have underlying diseases causing their manifestation. An example is primary hypertrophic osteoarthropathy, or pachydermoperiostosis. While clubbing makes up part of the clinical picture in both hypertrophic pulmonary osteoarthropathy and hypertrophic osteoarthropathy, the latter has no underlying disease associations. Rather, primary hypertrophic osteoarthropathy is familial, idiopathic, and has a chronic course often beginning during puberty in males. Secondary hypertrophic osteoarthropathy is an acquired form of clubbing that is classically associated with lung disease. However, it has also been associated with diseases of the heart, liver, and intestines. In the setting of pulmonary malignancy, secondary hypertrophic osteoarthropathy is known as hypertrophic pulmonary osteoarthropathy. Hypertrophic pulmonary osteoarthropathy has a distinct constellation of clinical findings that includes intractable pain often refractory to treatments other than resolution of the underlying disease process. The authors herein report a case of hypertrophic pulmonary osteoarthropathy masquerading as recurrent lower extremity cellulitis with chronic hand and foot pain in the setting of pulmonary malignancy that responded dramatically to intravenous Pamidronate Disodium (a bisphosphonate). Given the rarity of hypertrophic osteoarthropathy associated with lung cancer and the difficulty with pain management in such circumstances, the authors present the following case in which pain was mitigated by treatment with bisphosphonate therapy.
Efficacy and Safety of Zoledronic Acid and Pamidronate Disodium in the Treatment of Malignant Skeletal Metastasis: A Meta-Analysis.[Pubmed:26496320]
Medicine (Baltimore). 2015 Oct;94(42):e1822.
Solid tumors frequently metastasize to bone. Two bisphosphonates have been investigated for bone metastases including Pamidronate Disodium and zoledronic acid.By searching the PubMed, Embase, Wanfang, and China National Knowledge Infrastructure (CNKI) databases, we conducted a meta-analysis to determine the efficacy and safety of zoledronic acid compared with Pamidronate Disodium in reducing pain in patients with bone metastases.Studies were pooled, and the relative risk (RR) and its corresponding 95 % confidence interval (CI) were calculated. Version 12.0 STATA software was used for statistical analysis. Twenty relevant articles were included for this meta-analysis study.The complete response rate in cancer patients treatment with zoledronic acid was significantly higher than that with Pamidronate Disodium (relative risk [RR] = 1.32 [95% confidence interval (CI), 1.00-1.75]; P = 0.987, I = 0%). However, there was no significant difference in the rate of partial response rate (RR = 1.04, 95% CI: 0.90-1.20; P = 0.942, I = 0%) and in the total effective rate (RR = 1.06, 95% CI: 1.00-1.12; P = 0.998, I = 0%). For adverse events (AE), the incidence of headache in cancer patients with zoledronic acid was significantly lower than that with Pamidronate Disodium (RR = 0.82, 95% CI: 0.70-0.96; P = 0.793, I = 0%). There was no significant difference in nausea or vomiting (RR = 1.00, 95% CI: 0.92-1.09; P = 0.494, I = 0%), fever (RR = 0.98, 95% CI: 0.85-1.14; P =0.633, I = 0%), fatigue (RR = 1.01, 95% CI: 0.91-1.11; P = 0.914, I = 0%) and anorexia (RR = 1.31, 95% CI: 0.91-1.87; P = 0.024, I = 64.4%).In conclusion, this meta-analysis indicates that treatment with zoledronic acid was more effective than Pamidronate Disodium in the complete response assessments and the incidence of headache, an AE, was significantly lower in cancer patients with zoledronic acid.
Effects of pamidronate disodium on the loss of osteoarthritic subchondral bone and the expression of cartilaginous and subchondral osteoprotegerin and RANKL in rabbits.[Pubmed:25377946]
BMC Musculoskelet Disord. 2014 Nov 6;15:370.
BACKGROUND: Osteoarthritis (OA) is a major health problem in the increasingly elderly population. Therefore, it is crucial to prevent and treat OA at an early stage. The present study investigated whether Pamidronate Disodium (PAM), a bone-loss inhibitor, can significantly prevent or reverse the progression of early anterior cruciate ligament transection (ACLT)-induced OA. Whether therapeutic intervention is associated with regulation of the expression of osteoprotegerin (OPG), receptor activator of nuclear factor-kappaB ligand (RANKL), metalloproteinase-9 (MMP-9) or Toll-like receptor-4 (TLR-4) in cartilage and/or subchondral bone was also investigated. METHODS: 60 New Zealand rabbits were randomized into four groups: Sham-operated (n = 20); ACLT (n = 20); short-term treatment with PAM (PAM-S, n = 10) and long-term treatment with PAM (PAM-L, n = 10). For cartilage and subchondral bone testing, rabbits from Sham and ACLT groups were harvested at 2, 4, 6, and 14 weeks. Rabbits were given PAM from the 4th week after ACLT operation in PAM-S and PAM-L group, and were harvested at 6 and 14 weeks, respectively. Trabecular characteristics and cartilage changes were detected using Micro-CT, safranin O and rapid green staining, respectively. Immunohistochemical staining for OPG and RANKL were also performed. OPG, RANKL, MMP-9 and TLR-4 expression was evaluated by western blot analysis. RESULTS: Micro-CT and histology analyses indicated that PAM treatment for 2 or 10 weeks could completely prevent or reverse osteoarthritic subchondral bone loss and cartilage surface erosion. Immunohistochemistry and western blot analysis indicated that expression of OPG and RANKL increased, although RANKL expression increased more significantly than that of OPG. Therefore the ratio of OPG to RANKL was lower in the ACLT group. However, the ratio of OPG to RANKL in the PAM group was significantly higher than that in the ACLT group. Additionally, expression of MMP-9 and TLR-4 were upregulated in the ACLT group and downregulated in the PAM treated groups. CONCLUSIONS: PAM can significantly inhibit and even reverse early osteoarthritic subchondral bone loss, thus alleviating the process of cartilaginous degeneration. The mechanisms involved may be associated with the upregulation of OPG expression, and downregulation of RANKL, MMP-9 and TLR-4 expression.


