Cinnamyl alcoholCAS# 104-54-1 |
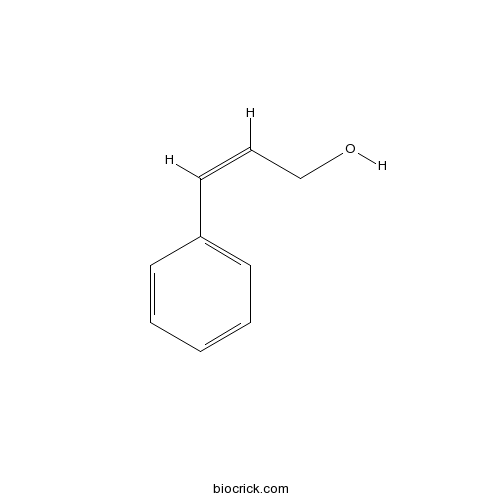
- 3-Phenyl-2-propen-1-ol
Catalog No.:BCN5493
CAS No.:4407-36-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 104-54-1 | SDF | Download SDF |
| PubChem ID | 5280511 | Appearance | Powder |
| Formula | C9H10O | M.Wt | 134.18 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (Z)-3-phenylprop-2-en-1-ol | ||
| SMILES | C1=CC=C(C=C1)C=CCO | ||
| Standard InChIKey | OOCCDEMITAIZTP-DAXSKMNVSA-N | ||
| Standard InChI | InChI=1S/C9H10O/c10-8-4-7-9-5-2-1-3-6-9/h1-7,10H,8H2/b7-4- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cinnamyl alcohol could as a fragrance ingredient with no safety concerns, it readily autoxidizes upon air exposure, and forms strong sensitizers as determined by the local lymph node assay. |
| In vitro | Cinnamyl alcohol oxidizes rapidly upon air exposure.[Pubmed: 23421457]Contact Dermatitis. 2013 Mar;68(3):129-38.Cinnamyl alcohol and cinnamal are frequent fragrance contact allergens. Both are included in the European baseline fragrance mix I, which is used for screening of contact allergy in dermatitis patients. The aim of this study was to investigate the autoxidation of Cinnamyl alcohol and to identify the oxidation products formed on air exposure. We also wanted to evaluate the effect of autoxidation on the sensitization potency of Cinnamyl alcohol. |
| In vivo | A toxicologic and dermatologic assessment of related esters and alcohols of cinnamic acid and cinnamyl alcohol when used as fragrance ingredients.[Pubmed: 18035463 ]Food Chem Toxicol. 2007;45 Suppl 1:S1-23.A toxicologic and dermatologic assessment of related esters and alcohols of cinnamic acid and Cinnamyl alcohol when used as fragrance ingredients. |
| Structure Identification | Chemistry. 2014 Apr 25;20(18):5478-86.Highly efficient direct aerobic oxidative esterification of cinnamyl alcohol with alkyl alcohols catalysed by gold nanoparticles incarcerated in a nanoporous polymer matrix: a tool for investigating the role of the polymer host.[Pubmed: 24644103]
|

Cinnamyl alcohol Dilution Calculator

Cinnamyl alcohol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 7.4527 mL | 37.2634 mL | 74.5268 mL | 149.0535 mL | 186.3169 mL |
| 5 mM | 1.4905 mL | 7.4527 mL | 14.9054 mL | 29.8107 mL | 37.2634 mL |
| 10 mM | 0.7453 mL | 3.7263 mL | 7.4527 mL | 14.9054 mL | 18.6317 mL |
| 50 mM | 0.1491 mL | 0.7453 mL | 1.4905 mL | 2.9811 mL | 3.7263 mL |
| 100 mM | 0.0745 mL | 0.3726 mL | 0.7453 mL | 1.4905 mL | 1.8632 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Anethole
Catalog No.:BCN5373
CAS No.:104-46-1
- 4-Methoxyphenylacetic acid
Catalog No.:BCN8467
CAS No.:104-01-8
- 4'-Hydroxypiptocarphin A
Catalog No.:BCN7113
CAS No.:103994-39-4
- 13-Hydroxygermacrone
Catalog No.:BCN3556
CAS No.:103994-29-2
- Ceftiofur hydrochloride
Catalog No.:BCC8911
CAS No.:103980-44-5
- Esculentic acid
Catalog No.:BCN5856
CAS No.:103974-74-9
- 15-Nor-14-oxolabda-8(17),12-dien-18-oic acid
Catalog No.:BCN1637
CAS No.:1039673-32-9
- Oleanolic acid 3-O-beta-D-glucosyl-(1->3)-alpha-L-rhamnosyl(1->2)-alpha-L-arabinoside
Catalog No.:BCN8132
CAS No.:103956-33-8
- Lupeol caffeate
Catalog No.:BCN5855
CAS No.:103917-26-6
- Nodosin
Catalog No.:BCN5854
CAS No.:10391-09-0
- (-)-Isodocarpin
Catalog No.:BCN3280
CAS No.:10391-08-9
- Maxacalcitol
Catalog No.:BCC1730
CAS No.:103909-75-7
- Cinnamaldehyde
Catalog No.:BCN6241
CAS No.:104-55-2
- Tussilagone
Catalog No.:BCN2770
CAS No.:104012-37-5
- 8alpha-Methacryloyloxybalchanin
Catalog No.:BCN4756
CAS No.:104021-39-8
- Trospium chloride
Catalog No.:BCC4582
CAS No.:10405-02-4
- LP533401 hcl
Catalog No.:BCC6377
CAS No.:1040526-12-2
- Quinovic acid 3-O-alpha-L-rhamnopyranoside
Catalog No.:BCN1636
CAS No.:104055-76-7
- Dihydroobovatin
Catalog No.:BCN3982
CAS No.:104055-79-0
- 3',5'-Diprenylgenistein
Catalog No.:BCN3572
CAS No.:104055-80-3
- Rehmapicroside
Catalog No.:BCN2884
CAS No.:104056-82-8
- Cyclo(Ile-Val)
Catalog No.:BCN2410
CAS No.:104068-43-1
- Atipamezole hydrochloride
Catalog No.:BCC7521
CAS No.:104075-48-1
- Zolantidine dimaleate
Catalog No.:BCC6922
CAS No.:104076-39-3
Cinnamyl alcohol oxidizes rapidly upon air exposure.[Pubmed:23421457]
Contact Dermatitis. 2013 Mar;68(3):129-38.
BACKGROUND: Cinnamyl alcohol and cinnamal are frequent fragrance contact allergens. Both are included in the European baseline fragrance mix I, which is used for screening of contact allergy in dermatitis patients. OBJECTIVES: The aim of this study was to investigate the autoxidation of Cinnamyl alcohol and to identify the oxidation products formed on air exposure. We also wanted to evaluate the effect of autoxidation on the sensitization potency of Cinnamyl alcohol. METHODS: Samples of commercially available Cinnamyl alcohol with and without purification were exposed to air, and the autoxidation was followed by chemical analysis. The analysis was performed with mass spectrometry (LC/MS/MS). Sensitization potencies of compounds were determined with the murine local lymph node assay (LLNA) in mice. RESULTS: Chemical analysis showed that the concentration of Cinnamyl alcohol in the air-exposed samples decreased rapidly over time, and that autoxidation products were formed. Cinnamal, epoxy Cinnamyl alcohol and cinnamic acid were identified as oxidation products. According to our study, cinnamal and epoxy Cinnamyl alcohol were the first autoxidation products formed. The epoxy Cinnamyl alcohol was shown to be the oxidation product with the highest sensitization potency. The analysis of our samples of commercially available Cinnamyl alcohol showed that there was already a content of 1.5% cinnamal at the start of the autoxidation experiments. CONCLUSION: Cinnamyl alcohol readily autoxidizes upon air exposure, and forms strong sensitizers as determined by the LLNA. Cinnamal was formed in the largest amounts, showing that cinnamal is not only formed via bioactivation, as has previously been shown. A highly sensitizing epoxide was also identified and quantified in the oxidation mixture.
Highly efficient direct aerobic oxidative esterification of cinnamyl alcohol with alkyl alcohols catalysed by gold nanoparticles incarcerated in a nanoporous polymer matrix: a tool for investigating the role of the polymer host.[Pubmed:24644103]
Chemistry. 2014 Apr 25;20(18):5478-86.
The selective aerobic oxidation of Cinnamyl alcohol to cinnamaldehyde, as well as direct oxidative esterification of this alcohol with primary and secondary aliphatic alcohols, were achieved with high chemoselectivity by using gold nanoparticles supported in a nanoporous semicrystalline multi-block copolymer matrix, which consisted of syndiotactic polystyrene-co-cis-1,4-polybutadiene. The cascade reaction that leads to the alkyl cinnamates occurs through two oxidation steps: the selective oxidation of Cinnamyl alcohol to cinnamaldehyde, followed by oxidation of the hemiacetal that results from the base-catalysed reaction of cinnamaldehyde with an aliphatic alcohol. The rate constants for the two steps were evaluated in the temperature range 10-45 degrees C. The Cinnamyl alcohol oxidation is faster than the oxidative esterification of cinnamaldehyde with methanol, ethanol, 2-propanol, 1-butanol, 1-hexanol or 1-octanol. The rate constants of the latter reaction are pseudo-zero order with respect to the aliphatic alcohol and decrease as the bulkiness of the alcohol is increased. The activation energy (Ea) for the two oxidation steps was calculated for esterification of Cinnamyl alcohol with 1-butanol (Ea = 57.8+/-11.5 and 62.7+/-16.7 kJ mol(-1) for the first and second step, respectively). The oxidative esterification of Cinnamyl alcohol with 2-phenylethanol follows pseudo-first-order kinetics with respect to 2-phenylethanol and is faster than observed for other alcohols because of fast diffusion of the aromatic alcohol in the crystalline phase of the support. The kinetic investigation allowed us to assess the role of the polymer support in the determination of both high activity and selectivity in the title reaction.
A toxicologic and dermatologic assessment of related esters and alcohols of cinnamic acid and cinnamyl alcohol when used as fragrance ingredients.[Pubmed:18035463]
Food Chem Toxicol. 2007;45 Suppl 1:S1-23.
An evaluation and review of a structurally related group of fragrance materials.


