CVT 10216Potent and selective ALDH2 inhibitor CAS# 1005334-57-5 |
- Celastrol
Catalog No.:BCN5986
CAS No.:34157-83-0
- Honokiol
Catalog No.:BCN1001
CAS No.:35354-74-6
- Deoxyarbutin
Catalog No.:BCC4774
CAS No.:53936-56-4
- EUK 134
Catalog No.:BCC4317
CAS No.:81065-76-1
- Edaravone
Catalog No.:BCC2480
CAS No.:89-25-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1005334-57-5 | SDF | Download SDF |
| PubChem ID | 23661666 | Appearance | Powder |
| Formula | C24H19NO7S | M.Wt | 465.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in 1eq. NaOH | ||
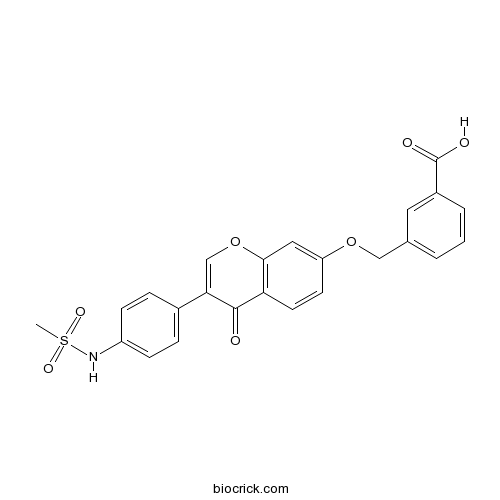
| Chemical Name | 3-[[3-[4-(methanesulfonamido)phenyl]-4-oxochromen-7-yl]oxymethyl]benzoic acid | ||
| SMILES | CS(=O)(=O)NC1=CC=C(C=C1)C2=COC3=C(C2=O)C=CC(=C3)OCC4=CC=CC(=C4)C(=O)O | ||
| Standard InChIKey | YYOOFJZTRCPVFD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H19NO7S/c1-33(29,30)25-18-7-5-16(6-8-18)21-14-32-22-12-19(9-10-20(22)23(21)26)31-13-15-3-2-4-17(11-15)24(27)28/h2-12,14,25H,13H2,1H3,(H,27,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective, reversible inhibitor of aldehyde dehydrogenase 2 (ALDH2) (IC50 values are 29 and 1300 nM for ALDH2 and ALDH1, respectively). Exhibits anxiolytic effects in rat models. |

CVT 10216 Dilution Calculator

CVT 10216 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1483 mL | 10.7416 mL | 21.4832 mL | 42.9664 mL | 53.708 mL |
| 5 mM | 0.4297 mL | 2.1483 mL | 4.2966 mL | 8.5933 mL | 10.7416 mL |
| 10 mM | 0.2148 mL | 1.0742 mL | 2.1483 mL | 4.2966 mL | 5.3708 mL |
| 50 mM | 0.043 mL | 0.2148 mL | 0.4297 mL | 0.8593 mL | 1.0742 mL |
| 100 mM | 0.0215 mL | 0.1074 mL | 0.2148 mL | 0.4297 mL | 0.5371 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Gelomulide N
Catalog No.:BCN6641
CAS No.:1005212-02-1
- Aeruginolactone
Catalog No.:BCN3695
CAS No.:1005208-88-7
- TCS 2002
Catalog No.:BCC6074
CAS No.:1005201-24-0
- Blasticidin A
Catalog No.:BCN1830
CAS No.:100513-53-9
- Sterigmatocystin
Catalog No.:BCN6885
CAS No.:10048-13-2
- Gastrin I (human)
Catalog No.:BCC5958
CAS No.:10047-33-3
- Rosiridin
Catalog No.:BCN5970
CAS No.:100462-37-1
- FFN 511
Catalog No.:BCC7799
CAS No.:1004548-96-2
- 1-EBIO
Catalog No.:BCC6904
CAS No.:10045-45-1
- Dihydroresveratrol 3-O-glucoside
Catalog No.:BCN5821
CAS No.:100432-87-9
- Cobicistat (GS-9350)
Catalog No.:BCC2271
CAS No.:1004316-88-4
- Boric acid
Catalog No.:BCC7592
CAS No.:10043-35-3
- LCL161
Catalog No.:BCC1691
CAS No.:1005342-46-0
- Tirasemtiv
Catalog No.:BCC5183
CAS No.:1005491-05-3
- Monomethylsulochrin
Catalog No.:BCN7255
CAS No.:10056-14-1
- TC ASK 10
Catalog No.:BCC6301
CAS No.:1005775-56-3
- TAK-593
Catalog No.:BCC5142
CAS No.:1005780-62-0
- NF 546
Catalog No.:BCC7804
CAS No.:1006028-37-0
- MK-2894
Catalog No.:BCC1757
CAS No.:1006036-87-8
- MK-2894 sodium salt
Catalog No.:BCC1758
CAS No.:1006036-88-9
- (-)-Epipinoresinol
Catalog No.:BCN3377
CAS No.:10061-38-8
- Desloratadine
Catalog No.:BCC4540
CAS No.:100643-71-8
- Ganoderenic acid A
Catalog No.:BCN3208
CAS No.:100665-40-5
- Ganoderenic acid B
Catalog No.:BCN7966
CAS No.:100665-41-6
Inhibition of aldehyde dehydrogenase-2 suppresses cocaine seeking by generating THP, a cocaine use-dependent inhibitor of dopamine synthesis.[Pubmed:20729865]
Nat Med. 2010 Sep;16(9):1024-8.
There is no effective treatment for cocaine addiction despite extensive knowledge of the neurobiology of drug addiction. Here we show that a selective aldehyde dehydrogenase-2 (ALDH-2) inhibitor, ALDH2i, suppresses cocaine self-administration in rats and prevents cocaine- or cue-induced reinstatement in a rat model of cocaine relapse-like behavior. We also identify a molecular mechanism by which ALDH-2 inhibition reduces cocaine-seeking behavior: increases in tetrahydropapaveroline (THP) formation due to inhibition of ALDH-2 decrease cocaine-stimulated dopamine production and release in vitro and in vivo. Cocaine increases extracellular dopamine concentration, which activates dopamine D2 autoreceptors to stimulate cAMP-dependent protein kinase A (PKA) and protein kinase C (PKC) in primary ventral tegmental area (VTA) neurons. PKA and PKC phosphorylate and activate tyrosine hydroxylase, further increasing dopamine synthesis in a positive-feedback loop. Monoamine oxidase converts dopamine to 3,4-dihydroxyphenylacetaldehyde (DOPAL), a substrate for ALDH-2. Inhibition of ALDH-2 enables DOPAL to condense with dopamine to form THP in VTA neurons. THP selectively inhibits phosphorylated (activated) tyrosine hydroxylase to reduce dopamine production via negative-feedback signaling. Reducing cocaine- and craving-associated increases in dopamine release seems to account for the effectiveness of ALDH2i in suppressing cocaine-seeking behavior. Selective inhibition of ALDH-2 may have therapeutic potential for treating human cocaine addiction and preventing relapse.
A selective ALDH-2 inhibitor reduces anxiety in rats.[Pubmed:19747934]
Pharmacol Biochem Behav. 2009 Dec;94(2):255-61.
CVT-10216 is a highly selective, reversible inhibitor of ALDH-2 that reduces excessive alcohol drinking. Anxiety plays a role in alcoholism. The present study asks whether CVT-10216 has anxiolytic properties, as reflected in social interaction behavior in four unrelated rodent models: endogenous anxiety-like behavior in naive Fawn-Hooded rats, repeated alcohol-withdrawal-induced anxiety, restraint stress-induced anxiety and drug-induced anxiety. CVT-10216 counteracted anxiety in all models except that produced by the 5-HT(2C) agonist, mCPP. CVT-10216 exhibited both acute and prophylactic inhibitions of repeated alcohol-withdrawal-induced anxiety. Importantly, anxiogenic behavior induced by the benzodiazepine receptor inverse agonist, DMCM, was counteracted dose-dependently by CVT-10216. Thus, a non-addictive selective inhibitor of ALDH-2 has both anxiolytic and antidipsotropic properties, which may be dependent, in part on the involvement of the GABA-benzodiazepine system.


