Gastrin I (human)Selective CCK2 agonist CAS# 10047-33-3 |
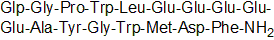
- Scrambled 10Panx
Catalog No.:BCC1246
CAS No.:1315378-72-3
- Gap 26
Catalog No.:BCC1032
CAS No.:197250-15-0
- 10Panx
Catalog No.:BCC1245
CAS No.:955091-53-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 10047-33-3 | SDF | Download SDF |
| PubChem ID | 16132267 | Appearance | Powder |
| Formula | C97H124N20O31S | M.Wt | 2098.22 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 50 mg/mL (23.83 mM) *"≥" means soluble, but saturation unknown. | ||
| Sequence | XGPWLEEEEEAYGWMDF (Modifications: X = Glp, Phe-17 = C-terminal amide) | ||
| SMILES | CC(C)CC(C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(CCC(=O)O)C(=O)NC(C)C(=O)NC(CC1=CC=C(C=C1)O)C(=O)NCC(=O)NC(CC2=CNC3=CC=CC=C32)C(=O)NC(CCSC)C(=O)NC(CC(=O)O)C(=O)NC(CC4=CC=CC=C4)C(=O)N)NC(=O)C(CC5=CNC6=CC=CC=C65)NC(=O)C7CCCN7C(=O)CNC(=O)C8CCC(=O)N8 | ||
| Standard InChIKey | GKDWRERMBNGKCZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C97H124N20O31S/c1-49(2)39-68(114-95(146)71(43-54-46-100-59-18-11-9-16-57(54)59)116-97(148)73-19-12-37-117(73)76(121)48-102-85(136)60-24-30-74(119)104-60)93(144)110-65(29-35-81(130)131)91(142)109-64(28-34-80(128)129)90(141)108-63(27-33-79(126)127)89(140)107-62(26-32-78(124)125)88(139)106-61(25-31-77(122)123)87(138)103-50(3)84(135)113-69(41-52-20-22-55(118)23-21-52)86(137)101-47-75(120)105-70(42-53-45-99-58-17-10-8-15-56(53)58)94(145)111-66(36-38-149-4)92(143)115-72(44-82(132)133)96(147)112-67(83(98)134)40-51-13-6-5-7-14-51/h5-11,13-18,20-23,45-46,49-50,60-73,99-100,118H,12,19,24-44,47-48H2,1-4H3,(H2,98,134)(H,101,137)(H,102,136)(H,103,138)(H,104,119)(H,105,120)(H,106,139)(H,107,140)(H,108,141)(H,109,142)(H,110,144)(H,111,145)(H,112,147)(H,113,135)(H,114,146)(H,115,143)(H,116,148)(H,122,123)(H,124,125)(H,126,127)(H,128,129)(H,130,131)(H,132,133) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous peptide produced in the stomach that acts as a selective CCK2 receptor agonist. Stimulates gastric acid secretion. |

Gastrin I (human) Dilution Calculator

Gastrin I (human) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Rosiridin
Catalog No.:BCN5970
CAS No.:100462-37-1
- FFN 511
Catalog No.:BCC7799
CAS No.:1004548-96-2
- 1-EBIO
Catalog No.:BCC6904
CAS No.:10045-45-1
- Dihydroresveratrol 3-O-glucoside
Catalog No.:BCN5821
CAS No.:100432-87-9
- Cobicistat (GS-9350)
Catalog No.:BCC2271
CAS No.:1004316-88-4
- Boric acid
Catalog No.:BCC7592
CAS No.:10043-35-3
- Lercanidipine
Catalog No.:BCC5239
CAS No.:100427-26-7
- Danshinspiroketallactone
Catalog No.:BCN3754
CAS No.:100414-80-0
- Sodium Picosulfate
Catalog No.:BCC4845
CAS No.:10040-45-6
- GSK 1562590 hydrochloride
Catalog No.:BCC8010
CAS No.:1003878-07-6
- Calcium chloride dihydrate
Catalog No.:BCC7582
CAS No.:10035-04-8
- Curcumenone
Catalog No.:BCN3008
CAS No.:100347-96-4
- Sterigmatocystin
Catalog No.:BCN6885
CAS No.:10048-13-2
- Blasticidin A
Catalog No.:BCN1830
CAS No.:100513-53-9
- TCS 2002
Catalog No.:BCC6074
CAS No.:1005201-24-0
- Aeruginolactone
Catalog No.:BCN3695
CAS No.:1005208-88-7
- Gelomulide N
Catalog No.:BCN6641
CAS No.:1005212-02-1
- CVT 10216
Catalog No.:BCC5606
CAS No.:1005334-57-5
- LCL161
Catalog No.:BCC1691
CAS No.:1005342-46-0
- Tirasemtiv
Catalog No.:BCC5183
CAS No.:1005491-05-3
- Monomethylsulochrin
Catalog No.:BCN7255
CAS No.:10056-14-1
- TC ASK 10
Catalog No.:BCC6301
CAS No.:1005775-56-3
- TAK-593
Catalog No.:BCC5142
CAS No.:1005780-62-0
- NF 546
Catalog No.:BCC7804
CAS No.:1006028-37-0
Pancreatic beta-Cells Express the Fetal Islet Hormone Gastrin in Rodent and Human Diabetes.[Pubmed:27864307]
Diabetes. 2017 Feb;66(2):426-436.
beta-Cell failure in type 2 diabetes (T2D) was recently proposed to involve dedifferentiation of beta-cells and ectopic expression of other islet hormones, including somatostatin and glucagon. Here we show that gastrin, a stomach hormone typically expressed in the pancreas only during embryogenesis, is expressed in islets of diabetic rodents and humans with T2D. Although gastrin in mice is expressed in insulin(+) cells, gastrin expression in humans with T2D occurs in both insulin(+) and somatostatin(+) cells. Genetic lineage tracing in mice indicates that gastrin expression is turned on in a subset of differentiated beta-cells after exposure to severe hyperglycemia. Gastrin expression in adult beta-cells does not involve the endocrine progenitor cell regulator neurogenin3 but requires membrane depolarization, calcium influx, and calcineurin signaling. In vivo and in vitro experiments show that gastrin expression is rapidly eliminated upon exposure of beta-cells to normal glucose levels. These results reveal the fetal hormone gastrin as a novel marker for reversible human beta-cell reprogramming in diabetes.
Gastrin decreases Na+,K+-ATPase activity via a PI 3-kinase- and PKC-dependent pathway in human renal proximal tubule cells.[Pubmed:26786777]
Am J Physiol Endocrinol Metab. 2016 Apr 1;310(7):E565-71.
The natriuretic effect of gastrin suggests a role in the coordinated regulation of sodium balance by the gastrointestinal tract and the kidney. The renal molecular targets and signal transduction pathways for such an effect of gastrin are largely unknown. Recently, we reported that gastrin induces NHE3 phosphorylation and internalization via phosphatidylinositol (PI) 3-kinase and PKCalpha. In this study, we show that gastrin induced the phosphorylation of human Na(+),K(+)-ATPase at serine 16, resulting in its endocytosis via Rab5 and Rab7 endosomes. The gastrin-stimulated phosphorylation of Na(+),K(+)-ATPase was dependent on PI 3-kinase because the phosphorylation was blocked by the PI 3-kinase inhibitor wortmannin. The phosphorylation of Na(+),K(+)-ATPase was also blocked by chelerythrine, a pan-PKC inhibitor, Go-6976, a conventional PKC (cPKC) inhibitor, and BAPTA-AM, an intracellular calcium chelator, suggesting the importance of cPKC and intracellular calcium in the gastrin signaling pathway. The gastrin-mediated phosphorylation of Na(+),K(+)-ATPase was also inhibited by U-73122, a phospholipase C (PLC) inhibitor. These results suggest that gastrin regulates sodium hydrogen exchanger and pump in renal proximal tubule cells at the apical and basolateral membranes.
Immunohistochemical examination of cholecystokinin and gastrin receptors (CCK-2/gastrin-R) expression in normal and exocrine cancerous human pancreatic tissues.[Pubmed:26520651]
Pancreatology. 2015 Nov-Dec;15(6):661-6.
OBJECTIVE: Evaluating tissue samples of normal and exocrine cancerous human pancreas on the expression of CCK2/gastrin receptor. We performed an immunohistochemical protocol that allows efficient detection of this receptor in formalin-fixed, paraffin-embedded human tissues. METHODS: Twenty (20) paraffin blocks of pancreatic tissue sections were collected from the Departments of pathology, Central University Hospital of Sidi-bel-Abbes City (Western Algeria) for the period 2004-2013; ten (10) of them were normal pancreatic samples; and ten (10) cancerous pancreatic sections. The samples were studied using an immunohistochemical protocol for CCK-2/gastrin receptors. RESULTS: Our immunohistochemical analysis revealed that CCK-2/gastrin receptors were expressed in both normal and malignant pancreatic cells but with different immunoreactivity levels and different immunostaining intensity i.e., CCK-2/gastrin receptors were highly expressed within the cytoplasmic area of cancerous cells; 40% of the samples had an immunoreactivity (IR) of (+++) and 60% (++++); the immunostaining was as well very intense since we reported a dark brown staining of the malignant cells. However; in normal pancreatic tissues; CCK-2/gastrin receptors IR levels were very low; 80% of the samples had an IR of (+); and 20% had (++) and the immunostaining was less intense; we noted a light brown staining of few normal pancreatic cells. CONCLUSION: The gastrointestinal peptides CCK could be very interesting targets for exocrine pancreatic cancer therapies; thus further surveys such as western blotting and RTPCR could indentify CCK-2/gastrin receptors as a helpful biomarker for exocrine pancreatic cancer diagnosis and treatment.
In Vitro Mouse and Human Serum Stability of a Heterobivalent Dual-Target Probe That Has Strong Affinity to Gastrin-Releasing Peptide and Neuropeptide Y1 Receptors on Tumor Cells.[Pubmed:28186846]
Cancer Biother Radiopharm. 2017 Feb;32(1):24-32.
Receptor-targeting radiolabeled molecular probes with high affinity and specificity are useful in studying and monitoring biological processes and responses. Dual- or multiple-targeting probes, using radiolabeled metal chelates conjugated to peptides, have potential advantages over single-targeting probes as they can recognize multiple targets leading to better sensitivity for imaging and radiotherapy when target heterogeneity is present. Two natural hormone peptide receptors, gastrin-releasing peptide (GRP) and Y1, are specifically interesting as their expression is upregulated in most breast and prostate cancers. One of our goals has been to develop a dual-target probe that can bind both GRP and Y1 receptors. Consequently, a heterobivalent dual-target probe, t-BBN/BVD15-DO3A (where a GRP targeting ligand J-G-Abz4-QWAVGHLM-NH2 and Y1 targeting ligand INP-K [varepsilon-J-(alpha-DO3A-varepsilon-DGa)-K] YRLRY-NH2 were coupled), that recognizes both GRP and Y1 receptors was synthesized, purified, and characterized in the past. Competitive displacement cell binding assay studies with the probe demonstrated strong affinity (IC50 values given in parentheses) for GRP receptors in T-47D cells (18 +/- 0.7 nM) and for Y1 receptors in MCF7 cells (80 +/- 11 nM). As a further evaluation of the heterobivalent dual-target probe t-BBN/BVD15-DO3A, the objective of this study was to determine its mouse and human serum stability at 37 degrees C. The in vitro metabolic degradation of the dual-target probe in mouse and human serum was studied by using a (153)Gd-labeled t-BBN/BVD15-DO3A and a high-performance liquid chromatography/radioisotope detector analytical method. The half-life (t1/2) of degradation of the dual-target probe in mouse serum was calculated as 7 hours and only approximately 20% degradation was seen after 6 hours incubation in human serum. The slow in vitro metabolic degradation of the dual-target probe can be compared with the degradation t1/2 of the corresponding monomeric probes, BVD15-DO3A and AMBA: 15, and approximately 40 minutes for BVD15-DO3A and 3.1 and 38.8 hours for AMBA in mouse and human serum, respectively. A possible pathway for in vitro metabolic degradation of the t-BBN/BVD15-DO3A in mouse serum is proposed based on the chromatographic retention times of the intact probe and its degradants.
CCK1 and CCK2 receptors regulate gastric pepsinogen secretion.[Pubmed:10408253]
Eur J Pharmacol. 1999 May 28;373(1):71-84.
The present study investigated (1) the pharmacological profile of cholecystokinin (CCK) receptor subtypes involved in the regulation of gastric pepsinogen secretion, (2) the influence of gastric acidity on peptic responses induced by CCK-8-sulfate (CCK-8S) or gastrin-I; and (3) the mechanisms accounting for the effects of CCK-like peptides on pepsinogen secretion. In anaesthetized rats, i.v. injection of CCK-8S or gastrin-I increased both pepsinogen and acid secretion. The pepsigogue effect of CCK-8S was higher than that of gastrin-I, whereas acid hypersecretion after CCK-8S was lower than that induced by gastrin-I. Peptic output following CCK-8S was partly blocked by i.v. injection of the CCK1 receptor antagonist, devazepide (-75.3%), or the CCK2 receptor antagonist, L-365,260 [3R(+)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4-benzodiazepine-3 yl)-N'-(3-methyl-phenyl)urea; -27.9%], but was fully prevented by combined administration of devazepide and L-365,260. The gastric acid hypersecretory effect of CCK-8S was enhanced by devazepide (+84.5%) and blocked by L-365,260. In contrast, the gastric secretory actions of gastrin-I were insensitive to devazepide, but abolished by L-365,260. Excitatory effects of CCK-8S and gastrin-I were not modified by vagotomy or atropine, whereas cimetidine or alpha-fluoromethylhistidine (irreversible blocker of histidine decarboxylase) partly prevented acid hypersecretion induced by both peptides without affecting their pepsigogue effects. After pretreatment with omeprazole, both CCK-8S and gastrin-I failed to stimulate acid secretion, while they increased pepsinogen output. In rats with gastric perfusion of acid solutions, CCK-8S or gastrin-I increased peptic output in a pH-independent manner either with or without pretreatment with omeprazole. Ablation of capsaicin-sensitive sensory nerves as well as application of lidocaine to the gastric mucosa failed to modify the excitatory effects of CCK-8S or gastrin-I on pepsinogen and acid secretion. Blockade of the nitric oxide (NO) synthase pathway by N(G)-nitro-L-arginine-methyl ester prevented the pepsigogue actions of both CCK-8S and gastrin-I (-61.8% and -71.7%, respectively), without affecting the concomitant increase in acid output. In addition, both these peptides significantly increased the release of NO breakdown products into the gastric lumen. The present results suggest that: (1) both CCK1 and CCK2 receptors mediate the peptic secretory responses induced by CCK-like peptides; (2) the excitatory inputs of CCK-8S and gastrin-I to chief cells are not driven through acid-dependent mechanisms or capsaicin-sensitive afferent sensory nerves; and (3) under in vivo conditions, the stimulant actions of CCK-like peptides on pepsinogen secretion are mediated, at least in part, by an increase in NO generation.


