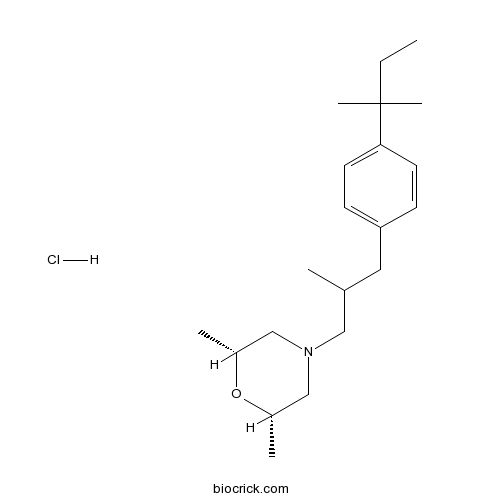
Amorolfine HClCAS# 78613-38-4 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78613-38-4 | SDF | Download SDF |
| PubChem ID | 54259 | Appearance | Powder |
| Formula | C21H36ClNO | M.Wt | 353.97 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 12.5 mg/mL (35.31 mM; Need ultrasonic) H2O : 3.33 mg/mL (9.41 mM; Need ultrasonic) | ||
| Chemical Name | (2R,6S)-2,6-dimethyl-4-[2-methyl-3-[4-(2-methylbutan-2-yl)phenyl]propyl]morpholine;hydrochloride | ||
| SMILES | [Cl-].CCC(C)(C)c1ccc(CC(C)CN2C[C@H](C)O[C@H](C)C2)cc1.[H+] | ||
| Standard InChIKey | XZKWIPVTHGWDCF-KUZYQSSXSA-N | ||
| Standard InChI | InChI=1S/C21H35NO.ClH/c1-7-21(5,6)20-10-8-19(9-11-20)12-16(2)13-22-14-17(3)23-18(4)15-22;/h8-11,16-18H,7,12-15H2,1-6H3;1H/t16?,17-,18+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Amorolfine HCl Dilution Calculator

Amorolfine HCl Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8251 mL | 14.1255 mL | 28.251 mL | 56.502 mL | 70.6275 mL |
| 5 mM | 0.565 mL | 2.8251 mL | 5.6502 mL | 11.3004 mL | 14.1255 mL |
| 10 mM | 0.2825 mL | 1.4125 mL | 2.8251 mL | 5.6502 mL | 7.0627 mL |
| 50 mM | 0.0565 mL | 0.2825 mL | 0.565 mL | 1.13 mL | 1.4125 mL |
| 100 mM | 0.0283 mL | 0.1413 mL | 0.2825 mL | 0.565 mL | 0.7063 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Amorolfine hydrochloride is a antifungal reagent.
- JDTic 2HCl
Catalog No.:BCC1671
CAS No.:785835-79-2
- Cycloastragenol
Catalog No.:BCN8483
CAS No.:78574-94-4
- FG 7142
Catalog No.:BCC6694
CAS No.:78538-74-6
- H-D-Ser(tBu)-OMe.HCl
Catalog No.:BCC3099
CAS No.:78537-14-1
- Excisanin A
Catalog No.:BCN7643
CAS No.:78536-37-5
- Excisanin B
Catalog No.:BCN4332
CAS No.:78536-36-4
- Dulcioic acid
Catalog No.:BCN4579
CAS No.:78516-69-5
- Asperumine
Catalog No.:BCN2039
CAS No.:78513-20-9
- PSB 069
Catalog No.:BCC7416
CAS No.:78510-31-3
- N-trans-Feruloyl-3-methoxytyramine
Catalog No.:BCN4331
CAS No.:78510-19-7
- 7-Epi-10-deacetylcephalomannine
Catalog No.:BCN7673
CAS No.:78479-12-6
- Praeruptorin E
Catalog No.:BCN2591
CAS No.:78478-28-1
- Terbinafine HCl
Catalog No.:BCC4863
CAS No.:78628-80-5
- Dehydroandrographolidesuccinate
Catalog No.:BCN8359
CAS No.:786593-06-4
- Z-D-Asp-OH
Catalog No.:BCC2786
CAS No.:78663-07-7
- 4,4'-Biphenyldicarboxylic acid
Catalog No.:BCC8655
CAS No.:787-70-2
- Plantagoside
Catalog No.:BCN8077
CAS No.:78708-33-5
- Ozagrel HCl
Catalog No.:BCC4926
CAS No.:78712-43-3
- D-AP4
Catalog No.:BCC6549
CAS No.:78739-01-2
- Shizukanolide C
Catalog No.:BCN6570
CAS No.:78749-47-0
- Flumazenil
Catalog No.:BCC1259
CAS No.:78755-81-4
- TC 1698 dihydrochloride
Catalog No.:BCC7394
CAS No.:787587-06-8
- Deapi-platycodin D
Catalog No.:BCN2614
CAS No.:78763-58-3
- Calcifediol-D6
Catalog No.:BCC4075
CAS No.:78782-98-6
In vitro studies with R 51,211 (itraconazole).[Pubmed:6089654]
Antimicrob Agents Chemother. 1984 Jul;26(1):5-9.
The in vitro activity of R 51,211 (itraconazole, accepted generic name; Janssen Pharmaceutica, Beerse, Belgium), a new orally active triazole, was compared with those of two existing orally active azoles, ketoconazole and BAY n 7133, and a topical agent, Ro 14-4767/002. An agar dilution procedure (Kimmig agar) was performed with 148 isolates of pathogenic fungi. Incubation was at 30 degrees C from 48 h to 7 days. R 51,211 was dissolved in 0.2 N HCl in absolute ethanol, ketoconazole was dissolved in 0.2 N HCl alone, BAY n 7133 was dissolved in absolute ethanol, and Ro 14-4767/002 was dissolved in dimethyl sulfoxide. R 51,211 and Ro 14-4767/002 were the most active drugs against isolates of Histoplasma capsulatum, and R 51,211 showed the greatest activity in vitro against isolates of Blastomyces dermatitidis and Cryptococcus neoformans. Ro 14-4767/002 was the most active drug against 30 isolates of dermatophytes, followed by R 51,211, ketoconazole, and BAY n 7133. R 51,211 showed the best activity in vitro against 19 isolates of Aspergillus fumigatus and Aspergillus flavus, as well as 19 isolates of dematiaceous fungi. All four drugs had 90% MICs of greater than or equal to 16 micrograms/ml when tested with isolates of zygomycetous fungi.
Application of Hansen Solubility Parameters to predict drug-nail interactions, which can assist the design of nail medicines.[Pubmed:26924329]
Eur J Pharm Biopharm. 2016 May;102:32-40.
We hypothesised that Hansen Solubility Parameters (HSPs) can be used to predict drug-nail affinities. Our aims were to: (i) determine the HSPs (deltaD, deltaP, deltaH) of the nail plate, the hoof membrane (a model for the nail plate), and of the drugs terbinafine HCl, Amorolfine HCl, ciclopirox olamine and efinaconazole, by measuring their swelling/solubility in organic liquids, (ii) predict nail-drug interactions by comparing drug and nail HSPs, and (iii) evaluate the accuracy of these predictions using literature reports of experimentally-determined affinities of these drugs for keratin, the main constituent of the nail plate and hoof. Many solvents caused no change in the mass of nail plates, a few solvents deswelled the nail, while others swelled the nail to varying extents. Fingernail and toenail HSPs were almost the same, while hoof HSPs were similar, except for a slightly lower deltaP. High nail-terbinafine HCl, nail-Amorolfine HCl and nail-ciclopirox olamine affinities, and low nail-efinaconazole affinities were then predicted, and found to accurately match experimental reports of these drugs' affinities to keratin. We therefore propose that drug and nail Hansen Solubility Parameters may be used to predict drug-nail interactions, and that these results can assist in the design of drugs for the treatment of nail diseases, such as onychomycosis and psoriasis. To our knowledge, this is the first report of the application of HSPs in ungual research.
UV-curable gel formulations: Potential drug carriers for the topical treatment of nail diseases.[Pubmed:26187167]
Int J Pharm. 2015 Aug 15;492(1-2):177-90.
Nail diseases are common, cause significant distress and treatments are far from successful. Our aim was to investigate the potential of UV-curable gels - currently used as cosmetics - as topical drug carriers for their treatment. These formulations have a long residence on the nail, which is expected to increase patient compliance and the success of topical therapy. The gels are composed of the diurethane dimethacrylate, ethyl methacrylate, 2-hydroxy-2-methylpropiophenone, an antifungal drug (Amorolfine HCl or terbinafine HCl) and an organic liquid (ethanol or NMP) as drug solvent. Following its application to a substrate and exposure to a UVA lamp for 2 min, the gel polymerises and forms a smooth, glossy and amorphous film, with negligible levels of residual monomers. No drug-polymer interactions were found and drug loading did not affect the film's properties, such as thickness, crystallinity and transition temperatures. In contrast, the organic solvent did influence the film's properties; NMP-containing films had lower glass transition temperatures, adhesion and water resistance than ethanol-based ones. Water-resistance being a desired property, ethanol-based formulations were investigated further for stability, drug release and ungual permeation. The films were stable under accelerated stability testing conditions. Compared to terbinafine, amorolfine was released to a greater extent, had a higher ungual flux, but a lower concentration in the nailplate. However, both drugs were present at considerably high levels in the nail when their MICs are taken into account. We thus conclude that UV-curable gels are promising candidates as topical nail medicines.


