N-trans-Feruloyl-3-methoxytyramineCAS# 78510-19-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78510-19-7 | SDF | Download SDF |
| PubChem ID | 5352115 | Appearance | Powder |
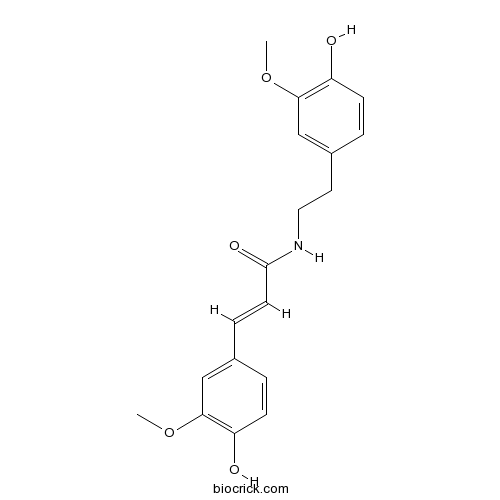
| Formula | C19H21NO5 | M.Wt | 343.4 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxy-3-methoxyphenyl)ethyl]prop-2-enamide | ||
| SMILES | COC1=C(C=CC(=C1)CCNC(=O)C=CC2=CC(=C(C=C2)O)OC)O | ||
| Standard InChIKey | GRXBVKANHNUZNL-VMPITWQZSA-N | ||
| Standard InChI | InChI=1S/C19H21NO5/c1-24-17-11-13(3-6-15(17)21)5-8-19(23)20-10-9-14-4-7-16(22)18(12-14)25-2/h3-8,11-12,21-22H,9-10H2,1-2H3,(H,20,23)/b8-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. N-trans-Feruloyl-3-methoxytyramine shows significantly DPPH radical scavenging activities. |

N-trans-Feruloyl-3-methoxytyramine Dilution Calculator

N-trans-Feruloyl-3-methoxytyramine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9121 mL | 14.5603 mL | 29.1206 mL | 58.2411 mL | 72.8014 mL |
| 5 mM | 0.5824 mL | 2.9121 mL | 5.8241 mL | 11.6482 mL | 14.5603 mL |
| 10 mM | 0.2912 mL | 1.456 mL | 2.9121 mL | 5.8241 mL | 7.2801 mL |
| 50 mM | 0.0582 mL | 0.2912 mL | 0.5824 mL | 1.1648 mL | 1.456 mL |
| 100 mM | 0.0291 mL | 0.1456 mL | 0.2912 mL | 0.5824 mL | 0.728 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-Epi-10-deacetylcephalomannine
Catalog No.:BCN7673
CAS No.:78479-12-6
- Praeruptorin E
Catalog No.:BCN2591
CAS No.:78478-28-1
- Enterolakton
Catalog No.:BCC8170
CAS No.:78473-71-9
- 7-Epi 10-Desacetyl Paclitaxel
Catalog No.:BCC1314
CAS No.:78454-17-8
- 19-Hydroxybaccatin III
Catalog No.:BCN4330
CAS No.:78432-78-7
- Deacetyltaxol
Catalog No.:BCN2820
CAS No.:78432-77-6
- RGB-286638 free base
Catalog No.:BCC5520
CAS No.:784210-88-4
- RGB-286638
Catalog No.:BCC5519
CAS No.:784210-87-3
- 5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavone
Catalog No.:BCN1353
CAS No.:78417-26-2
- Trequinsin hydrochloride
Catalog No.:BCC7333
CAS No.:78416-81-6
- Milrinone
Catalog No.:BCC4374
CAS No.:78415-72-2
- 1,7-Dihydroxy-2,3-dimethoxyxanthone
Catalog No.:BCN7523
CAS No.:78405-33-1
- PSB 069
Catalog No.:BCC7416
CAS No.:78510-31-3
- Asperumine
Catalog No.:BCN2039
CAS No.:78513-20-9
- Dulcioic acid
Catalog No.:BCN4579
CAS No.:78516-69-5
- Excisanin B
Catalog No.:BCN4332
CAS No.:78536-36-4
- Excisanin A
Catalog No.:BCN7643
CAS No.:78536-37-5
- H-D-Ser(tBu)-OMe.HCl
Catalog No.:BCC3099
CAS No.:78537-14-1
- FG 7142
Catalog No.:BCC6694
CAS No.:78538-74-6
- Cycloastragenol
Catalog No.:BCN8483
CAS No.:78574-94-4
- JDTic 2HCl
Catalog No.:BCC1671
CAS No.:785835-79-2
- Amorolfine HCl
Catalog No.:BCC4889
CAS No.:78613-38-4
- Terbinafine HCl
Catalog No.:BCC4863
CAS No.:78628-80-5
- Dehydroandrographolidesuccinate
Catalog No.:BCN8359
CAS No.:786593-06-4
[Phenylpropanoid amides from whole plants of Corydalis edulis].[Pubmed:29552819]
Zhongguo Zhong Yao Za Zhi. 2018 Jan;43(1):109-113.
Ten phenylpropanoid amides were isolated from the whole plants of Corydalis edulis Maxim. by various of column chromatographies including silica gel, Sephadex LH-20, and ODS. Their structures were identified on the basis of physicochemical properties, MS, NMR, and IR spectroscopic data. These compounds were identified as N-trans-sinapoyl-3-methoxytyramine-4'-O-beta-glucoside(1), N-trans-sinapoyl-3-methoxytyramine(2), N-trans-sinapoyltyramine(3), N-trans-p-coumaroyltyramine(4), N-trans-sinapoyl-7-hydroxytyramine(5), N-cis-feruloyltyramine(6), N-cis-p-coumaroyltyramine(7), N-trans-feruloyltyramine(8), N-trans-Feruloyl-3-methoxytyramine(9), and N-trans-feruloyl-7-hydroxytyramine(10). Compound 1 is a new compound. Compounds 2-7 are obtained from the plants of Papaveraceae for the first time, while compounds 8-10 are firstly isolated from C. edulis.
An isoindole alkaloid from Portulaca oleracea L.[Pubmed:29272980]
Nat Prod Res. 2018 Oct;32(20):2431-2436.
A novel isoindole alkaloid named oleraisoindole (1), together with six known compounds, 7'-ethoxy-trans-feruloyltyramine (2), N-trans-feruloyltyramine (3), N-trans-Feruloyl-3-methoxytyramine (4), N-trans-p-coumaroyltyramine (5) aurantiamide (6) and ferulic acid methyl ester (7) were isolated from Portulaca oleracea L. Compounds 2 and 7 were isolated for the first time from this plant. Compound 1 was identified using spectroscopic methods including HR-ESI-TOF-MS, 1D-NMR, 2D-NMR. It was tested in a nitric oxide (NO) inhibition assay and was shown to inhibit NO production in RAW 264.7 cells induced by LPS.


