AmentoflavoneCAS# 1617-53-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1617-53-4 | SDF | Download SDF |
| PubChem ID | 5281600 | Appearance | Light yellow powder |
| Formula | C30H18O10 | M.Wt | 538.46 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Didemethyl-ginkgetin | ||
| Solubility | DMSO : ≥ 34 mg/mL (63.14 mM) *"≥" means soluble, but saturation unknown. | ||
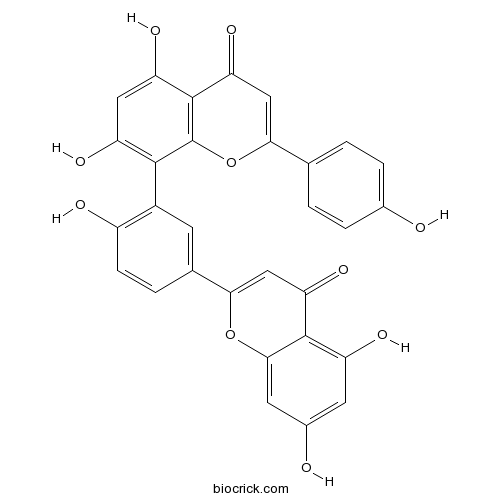
| Chemical Name | 8-[5-(5,7-dihydroxy-4-oxochromen-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2=CC(=O)C3=C(O2)C(=C(C=C3O)O)C4=C(C=CC(=C4)C5=CC(=O)C6=C(C=C(C=C6O5)O)O)O)O | ||
| Standard InChIKey | YUSWMAULDXZHPY-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Amentoflavone is a novel natural inhibitor of human Cathepsin B(CatB), which has antifungal , antioxidant, antiviral, antidiabetic, and neuroprotective activities, it stimulates apoptosis in HSFBs and inhibits angiogenesis of endothelial cells, it is a promising molecule that can be used in hypertrophic scar treatment. Amentoflavone regulated β-catenin and caspase-3 expressions, and inhibited NF-κB signal transduction pathways. |
| Targets | NO | TNF-α | NOS | COX | NF-kB | Akt | mTOR | JNK | Caspase | VEGFR | IL Receptor |
| In vitro | Fatty acid synthase inhibition by amentoflavone suppresses HER2/neu (erbB2) oncogene in SKBR3 human breast cancer cells.[Pubmed: 22767439 ]Phytother Res. 2013 May;27(5):713-20.Fatty acid synthase (FASN) is a potential therapeutic target for treatment of cancer and obesity, and is highly elevated in 30% of HER2-overexpressing breast cancers. Considerable interest has developed in searching for novel FASN inhibitors as therapeutic agents in treatment of HER2-overexpressing breast cancers.
Amentoflavone inhibits angiogenesis of endothelial cells and stimulates apoptosis in hypertrophic scar fibroblasts.[Pubmed: 24280521]Burns. 2014 Aug;40(5):922-9.Amentoflavone (8-[5-(5,7-dihydroxy-4-oxo-chromen-2-yl)-2-hydroxy-phenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl) chromen-4-one; AF) is a biflavonoid derived from the extracts of Selaginella tamariscina. It has been shown that AF has diverse biological effects such as antitumour, etc. It is well known that high cell proliferation, viability, angiogenesis and low apoptosis are key factors in hypertrophic scar formation.
Antifungal effect of amentoflavone derived from Selaginella tamariscina.[Pubmed: 17024847]Arch Pharm Res. 2006 Sep;29(9):746-51.Amentoflavone is a plant bif avonoid that was isolated from an ethyl acetate extract of the whole plant of Selaginella tamariscina (Beauv.) spring. 1D and 2D NMR spectroscopy including DEPT, HMQC, and HMBC were used to determine its structure.
|
| In vivo | Amentoflavone inhibits iNOS, COX-2 expression and modulates cytokine profile, NF-κB signal transduction pathways in rats with ulcerative colitis.[Pubmed: 24126114]Int Immunopharmacol. 2013 Nov;17(3):907-16.Ulcerative colitis is a chronic inflammatory disorder characterized by oxidative stress, leucocyte infiltration and upregulation of pro-inflammatory cytokines.
|
| Kinase Assay | Amentoflavone and its derivatives as novel natural inhibitors of human Cathepsin B.[Pubmed: 16084098 ]Bioorg Med Chem. 2005 Oct 15;13(20):5819-25.Cathepsin B (CatB) is a member of the papain superfamily of cysteine proteases and has been implicated in the pathology of numerous diseases, including arthritis and cancer. Amentoflavone is found in a number of plants with medicinal properties, including Ginkgo biloba and Hypericum perforatum (St. John's Wort).
|
| Cell Research | [Amentoflavone induces apoptosis in SW480 human colorectal cancer cells via regulating β-catenin and caspase-3 expressions].[Pubmed: 25057079 ]Nan Fang Yi Ke Da Xue Xue Bao. 2014 Jun;34(7):1035-8.To investigate the role of β-catenin and caspase-3 in Amentoflavone-induced apoptosis of human colorectal cancer SW480 cells.
|
| Animal Research | Inhibition of tumor specific angiogenesis by amentoflavone.[Pubmed: 18298378]Biochemistry (Mosc). 2008 Feb;73(2):209-18.The formation of new capillaries from existing blood vessels is critical for tumor growth and metastasis. In this study we report that Amentoflavone, a biflavonoid from Biophytum sensitivum, could inhibit the process of angiogenesis.
|

Amentoflavone Dilution Calculator

Amentoflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8571 mL | 9.2857 mL | 18.5715 mL | 37.143 mL | 46.4287 mL |
| 5 mM | 0.3714 mL | 1.8571 mL | 3.7143 mL | 7.4286 mL | 9.2857 mL |
| 10 mM | 0.1857 mL | 0.9286 mL | 1.8571 mL | 3.7143 mL | 4.6429 mL |
| 50 mM | 0.0371 mL | 0.1857 mL | 0.3714 mL | 0.7429 mL | 0.9286 mL |
| 100 mM | 0.0186 mL | 0.0929 mL | 0.1857 mL | 0.3714 mL | 0.4643 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Amentoflavone is a natural biflavone compound with many biological properties, including anti-inflammatory, antioxidative, and neuroprotective effects. IC50 value: Target: In vitro: In irradiated v79 cells, Pretreatment with amentoflavone 24 hours prior to 8 Gy 60Co γ-ray irradiation significantly inhibited apoptosis, promoted the G2 phase, decreased the concentration of ROS and mitochondrial mass [2]. Amentoflavone dose-dependently inhibited the viability of SW480 cells, and a high concentration of amentoflavone (150 μmol/L) obviously induced apoptosis of the cells [3]. In vivo: In epilepsy models, amentoflavone effectively prevented pilocarpine-induced epilepsy in a mouse kindling model, suppressed nuclear factor-κB activation and expression, inhibited excessive discharge of hippocampal neurons resulting in a reduction in epileptic seizures, shortened attack time, and diminished loss and apoptosis of hippocampal neurons [1].
References:
[1]. Zhang Z, et al. Amentoflavone protects hippocampal neurons: anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen Res. 2015 Jul;10(7):1125-33.
[2]. Xu P, et al. Amentoflavone acts as a radioprotector for irradiated v79 cells by regulating reactive oxygen species (ROS), cell cycle and mitochondrial mass. Asian Pac J Cancer Prev. 2014;15(18):7521-6.
[3]. Yang Y, et al. [Amentoflavone induces apoptosis in SW480 human colorectal cancer cells via regulating β-catenin and caspase-3 expressions]. Nan Fang Yi Ke Da Xue Xue Bao. 2014 Jun;34(7):1035-8.
- 2,3,8-Tri-O-methylellagic acid
Catalog No.:BCN1716
CAS No.:1617-49-8
- Caesalpine B
Catalog No.:BCN7377
CAS No.:1616757-60-8
- Caesalpine A
Catalog No.:BCN7376
CAS No.:1616757-59-5
- Dodovislactone B
Catalog No.:BCN7398
CAS No.:1616683-55-6
- Dodovislactone A
Catalog No.:BCN7399
CAS No.:1616683-54-5
- Dodovisone D
Catalog No.:BCN6871
CAS No.:1616683-53-4
- Dodovisone C
Catalog No.:BCN6872
CAS No.:1616683-52-3
- Dodovisone B
Catalog No.:BCN6867
CAS No.:1616683-51-2
- Dodovisone A
Catalog No.:BCN6839
CAS No.:1616683-50-1
- ONC201
Catalog No.:BCC3989
CAS No.:1616632-77-9
- Erythrinin H
Catalog No.:BCN6870
CAS No.:1616592-62-1
- Erythrinin G
Catalog No.:BCN6857
CAS No.:1616592-61-0
- Lupeol acetate
Catalog No.:BCN6893
CAS No.:1617-68-1
- Lupenone
Catalog No.:BCN1717
CAS No.:1617-70-5
- Vincamine
Catalog No.:BCN2606
CAS No.:1617-90-9
- Z-Ile-Leu-aldehyde
Catalog No.:BCC5591
CAS No.:161710-10-7
- Tebipenem
Catalog No.:BCC5550
CAS No.:161715-21-5
- Tebipenempivoxil
Catalog No.:BCC3861
CAS No.:161715-24-8
- Rasagiline mesylate
Catalog No.:BCN2166
CAS No.:161735-79-1
- GLP-1 (9-36) amide
Catalog No.:BCC6001
CAS No.:161748-29-4
- 12-Oxocalanolide A
Catalog No.:BCN4699
CAS No.:161753-49-7
- Palmatrubine
Catalog No.:BCN2647
CAS No.:16176-68-4
- Esomeprazole Sodium
Catalog No.:BCC4376
CAS No.:161796-78-7
- Ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate
Catalog No.:BCC8969
CAS No.:161797-99-5
Antifungal effect of amentoflavone derived from Selaginella tamariscina.[Pubmed:17024847]
Arch Pharm Res. 2006 Sep;29(9):746-51.
Amentoflavone is a plant bif avonoid that was isolated from an ethyl acetate extract of the whole plant of Selaginella tamariscina (Beauv.) spring. 1D and 2D NMR spectroscopy including DEPT, HMQC, and HMBC were used to determine its structure. Amentoflavone exhibited potent antifungal activity against several pathogenic fungal strains but had a very low hemolytic effect on human erythrocytes. In particular, Amentoflavone induced the accumulation of intracellular trehalose on C. albicans as a stress response to the drug, and disrupted the dimorphic transition that forms pseudo-hyphae during pathogenesis. In conclusion, Amentoflavone has great potential to be a lead compound for the development of antifungal agents.
Amentoflavone inhibits iNOS, COX-2 expression and modulates cytokine profile, NF-kappaB signal transduction pathways in rats with ulcerative colitis.[Pubmed:24126114]
Int Immunopharmacol. 2013 Nov;17(3):907-16.
Ulcerative colitis is a chronic inflammatory disorder characterized by oxidative stress, leucocyte infiltration and upregulation of pro-inflammatory cytokines. The aim of the present study was to examine the effect of Amentoflavone on a murine model of ulcerative colitis (UC). UC was induced by intracolonic injection of 3% acetic acid in male Wistar rats. Amentoflavone (10 mg/kg.b.wt) or reference drug sulfasalazine (100 mg/kg.b.wt) was administrated intra-peritoneally for 5 consecutive days before induction of colitis with acetic acid. Administration of Amentoflavone was found to reduce the extent of inflammatory colonic injury. This was manifested by a decrease in the score of mucosal injury, by lowered colonic wet weight as well as vascular permeability and diminished lactate dehydrogenase (LDH) and myeloperoxidase (MPO) activity reflecting reduced leukocyte infiltration. Furthermore, the mucosal content of lipid peroxidation (LPO), glutathione (GSH), superoxide dismutase (SOD), nitric oxide (NO) activity confirms that Amentoflavone could significantly inhibit colitis. The treatment also reduced significantly the colonic tumor necrosis factor-alpha (TNF-alpha), interleukin-1 beta (IL-1beta) and IL-6 levels as well as the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) compared to colitis control group. The histopathological studies also confirm the foregoing findings. Amentoflavone was also able to inhibit the activation and translocation of transcription factors, nuclear factor (NF)-kappaB subunits (p65/p50). These results suggest that Amentoflavone exhibits protective effect in acetic acid-induced ulcerative colitis which might be due to its modulation of oxidant/anti-oxidant balance, down-regulation of productions and expressions of pro-inflammatory cytokines, inflammatory mediators and inhibition of NF-kappaB signal transduction pathways.
Inhibition of tumor specific angiogenesis by amentoflavone.[Pubmed:18298378]
Biochemistry (Mosc). 2008 Feb;73(2):209-18.
The formation of new capillaries from existing blood vessels is critical for tumor growth and metastasis. In this study we report that Amentoflavone, a biflavonoid from Biophytum sensitivum, could inhibit the process of angiogenesis. Amentoflavone at nontoxic concentrations (0.05-0.2 microg/ml) showed significant inhibition in the proliferation, migration, and tube formation of endothelial cells, which are key events in the process of angiogenesis. In vivo studies in C57BL/6 mice using Amentoflavone showed remarkable inhibition (52.9%) of tumor directed capillary formation. Amentoflavone showed inhibitory effect on the production of various endogenous factors such as IL-1beta, IL-6, TNF-alpha, GM-CSF, and VEGF that control the process of angiogenesis. Amentoflavone treatment could increase the production of IL-2 and TIMP-1, which could successfully shift the equilibrium towards an angiostatic condition. The antiangiogenic activity of Amentoflavone was supported by its remarkable suppression in sprouting of microvessels from rat aorta. Our results also show that Amentoflavone could inhibit the production of VEGF mRNA in B16-F10 cells. These findings indicate that Amentoflavone inhibits angiogenesis by disrupting the integrity of endothelial cells and by altering the endogenous factors that are required for the process of neovascularization.
Amentoflavone and its derivatives as novel natural inhibitors of human Cathepsin B.[Pubmed:16084098]
Bioorg Med Chem. 2005 Oct 15;13(20):5819-25.
Cathepsin B (CatB) is a member of the papain superfamily of cysteine proteases and has been implicated in the pathology of numerous diseases, including arthritis and cancer. Amentoflavone is found in a number of plants with medicinal properties, including Ginkgo biloba and Hypericum perforatum (St. John's Wort). Herein, we report the structure-activity relationship (SAR) and binding mechanism of three biflavones, Amentoflavone (AMF1), 4'''-methylAmentoflavone (AMF2) and 7'',4'''-dimethylAmentoflavone (AMF3), isolated from Taxodium mucronatum by us as novel natural inhibitors of human CatB with strong inhibitory activities at IC50 values of 1.75, 1.68 and 0.55muM, respectively. Density functional theory (DFT) method was applied to optimize the geometry structures of AMF1, AMF2 and AMF3 at the B3LYP/6-31G* level. FlexX was explored to dock the three biflavones to the binding sites of CatB, and to get a better understanding of vital interactions between these biflavones and CatB. A good correlation between the calculated quantum descriptors and the experimental inhibitory activities suggested that quantum model of these potential inhibitors is reliable. Through geometry and electron structure analysis of AMFs, it was observed that the CH3 substitute at 7'' and 4''' positions could not vary the difference in geometry structure significantly, but increase the electron density of A-ring, HOMO energy, hydrophobic property, and improve inhibitory activity. Structural and energetic analysis of AMFs and AMFs-CatB complexes showed that the electron-donor site is the A-ring, which shows the highest HOMO energy distribution, and the electron-acceptor site is the F-ring, which shows the highest LUMO energy distribution in AMFs, and the pi-pi interaction between A-ring and residue Trp221, two hydrogen bonds (O5 and Trp221; O4 and Gln23 ), hydrophobic interaction between the C-ring and residue Cys29 and CH3 substitutes at 7'' and 4''' might play a crucial role in the inhibition of AMFs on CatB. Results indicated that AMFs are new natural reversible inhibitors that would be useful in developing potent inhibitors of CatB.
Fatty acid synthase inhibition by amentoflavone suppresses HER2/neu (erbB2) oncogene in SKBR3 human breast cancer cells.[Pubmed:22767439]
Phytother Res. 2013 May;27(5):713-20.
Fatty acid synthase (FASN) is a potential therapeutic target for treatment of cancer and obesity, and is highly elevated in 30% of HER2-overexpressing breast cancers. Considerable interest has developed in searching for novel FASN inhibitors as therapeutic agents in treatment of HER2-overexpressing breast cancers. Amentoflavone was found to be effective in suppressing FASN expression in HER2-positive SKBR3 cells. Pharmacological inhibition of FASN by Amentoflavone specifically down-regulated HER2 protein and mRNA, and caused an up-regulation of PEA3, a transcriptional repressor of HER2. In addition, pharmacological blockade of FASN by Amentoflavone preferentially decreased cell viability and induced cell death in SKBR3 cells. Palmitate reduced the cytotoxic effect of Amentoflavone, as the percentage of viable cells was increased after the addition of exogenous palmitate. Amentoflavone-induced FASN inhibition inhibited the translocation of SREBP-1 in SKBR3 cells. Amentoflavone inhibited phosphorylation of AKT, mTOR, and JNK. The use of pharmacological inhibitors revealed that the modulation of AKT, mTOR, and JNK phosphorylation required synergistic Amentoflavone-induced FASN inhibition and HER2 activation in SKBR3 cells. These results suggest that Amentoflavone modulated FASN expression by regulation of HER2-pathways, and induced cell death to enhance chemopreventive or chemotherapeutic activity in HER2-positive breast cancers.
[Amentoflavone induces apoptosis in SW480 human colorectal cancer cells via regulating beta-catenin and caspase-3 expressions].[Pubmed:25057079]
Nan Fang Yi Ke Da Xue Xue Bao. 2014 Jun;34(7):1035-8.
OBJECTIVE: To investigate the role of beta-catenin and caspase-3 in Amentoflavone-induced apoptosis of human colorectal cancer SW480 cells. METHODS: MTT assay was used to detect the viability of SW480 cells exposed to Amentoflavone, and flow cytometry was employed to assess the cell apoptosis. Western blotting was performed to determine the protein expressions of beta-catenin and caspase-3 in the exposed cells. RESULTS: Amentoflavone dose-dependently inhibited the viability of SW480 cells, and a high concentration of Amentoflavone (150 micromol/L) obviously induced apoptosis of the cells. Amentoflavone exposure caused significantly increased expression of caspase-3 and suppressed beta-catenin expression in the cells. CONCLUSION: Amentoflavone-induced apoptosis in SW480 human colorectal cancer cells is associated with altered expressions of beta-catenin and caspase-3.
Amentoflavone inhibits angiogenesis of endothelial cells and stimulates apoptosis in hypertrophic scar fibroblasts.[Pubmed:24280521]
Burns. 2014 Aug;40(5):922-9.
Amentoflavone (8-[5-(5,7-dihydroxy-4-oxo-chromen-2-yl)-2-hydroxy-phenyl]-5,7-dihydroxy-2-(4-hyd roxyphenyl) chromen-4-one; AF) is a biflavonoid derived from the extracts of Selaginella tamariscina. It has been shown that AF has diverse biological effects such as antitumour, etc. It is well known that high cell proliferation, viability, angiogenesis and low apoptosis are key factors in hypertrophic scar formation. In this study, we report that AF inhibited viability and stimulated apoptosis in hypertrophic scar fibroblasts (HSFBs). Incubation of HSFBs with AF showed its inhibitory effect on cell viability and the exhibition of a series of cellular changes that were consistent with apoptosis. By Western-blot analysis, our data indicated significant increases in the amounts of cleaved caspases 3, 8, 9 and Bax, several apoptotic promoters and a significant decrease in translationally controlled tumour protein (TCTP), an apoptotic inhibitor, in HSFBs treated with AF. Furthermore, AF showed significant inhibitions on the viability, migration and tube formation of endothelial cells, which are associated with angiogenesis. In conclusion, this study suggests that AF stimulates apoptosis in HSFBs and inhibits angiogenesis of endothelial cells. Therefore, AF is a promising molecule that can be used in hypertrophic scar treatment.


