AH 11110 hydrochlorideα1B-AR ligand,subtype-selective CAS# 179388-65-9 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 179388-65-9 | SDF | Download SDF |
| PubChem ID | 9907616 | Appearance | Powder |
| Formula | C21H27ClN2O2 | M.Wt | 374.91 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in water | ||
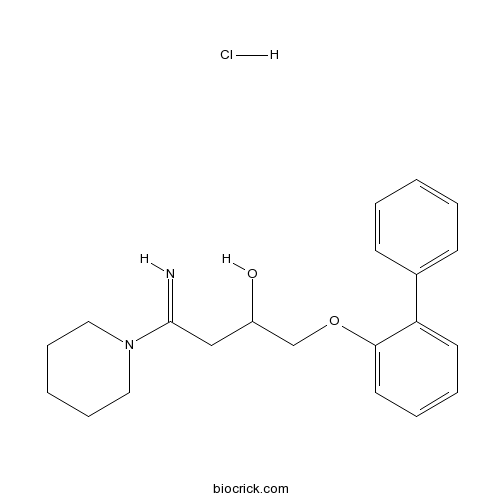
| Chemical Name | 4-imino-1-(2-phenylphenoxy)-4-piperidin-1-ylbutan-2-ol;hydrochloride | ||
| SMILES | C1CCN(CC1)C(=N)CC(COC2=CC=CC=C2C3=CC=CC=C3)O.Cl | ||
| Standard InChIKey | WWHGFIXWBLHHQB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H26N2O2.ClH/c22-21(23-13-7-2-8-14-23)15-18(24)16-25-20-12-6-5-11-19(20)17-9-3-1-4-10-17;/h1,3-6,9-12,18,22,24H,2,7-8,13-16H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A subtype-selective ligand for the α1B-adrenoceptor. The pKi values for binding to rat fibroblast cell membranes expressing α1B (hamster), α1A (bovine) and α1D (rat) are 7.1, 5.59, and 5.68 respectively. Appears to be non-selective between α1 subtypes and α2 receptors in smooth muscle preparations. |

AH 11110 hydrochloride Dilution Calculator

AH 11110 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6673 mL | 13.3365 mL | 26.6731 mL | 53.3461 mL | 66.6827 mL |
| 5 mM | 0.5335 mL | 2.6673 mL | 5.3346 mL | 10.6692 mL | 13.3365 mL |
| 10 mM | 0.2667 mL | 1.3337 mL | 2.6673 mL | 5.3346 mL | 6.6683 mL |
| 50 mM | 0.0533 mL | 0.2667 mL | 0.5335 mL | 1.0669 mL | 1.3337 mL |
| 100 mM | 0.0267 mL | 0.1334 mL | 0.2667 mL | 0.5335 mL | 0.6668 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AH 11110 hydrochloride is a selective antagonist of α1B-adrenoceptor [1].
α1B-adrenoceptor (α1B-AR) belongs to α1- adrenergic receptors, which include three subtypes α1A, α1B and α1D and play an important role in regulating cell growth and proliferation.
AH 11110 hydrochloride is a selective α1B-AR antagonist. AH11110A inhibited cloned α1B-AR with Ki value of 79.4 nM and exhibited 32- and 26-fold selectivity over α1A- and α1D-ARs, respectively [1]. In rat vas deferens and rat perfused kidney, AH11110A exhibited affinities for α1A-AR, α1B-AR and α1D-AR with pA2 values of 6.41, 5.40-6.54 and 5.47-5.48, respectively. Also, AH11110A displayed affinity for α2-adrenoceptor in rabbit vas deferens with pA2 value of 5.44. In ligand binding studies, AH11110A exhibited affinities for native and cloned α1B-AR with pKi of 7.10-7.73. AH11110A increased contraction of rat vas deferens [2]. In isolated spleen strips from guinea-pig, AH11110A competitively antagonized tissue contraction induced by noradrenaline in a concentration-dependent way [2].
References:
[1]. Erami C, Zhang H, Ho JG, et al. Alpha(1)-adrenoceptor stimulation directly induces growth of vascular wall in vivo. Am J Physiol Heart Circ Physiol, 2002, 283(4): H1577-1587.
[2]. Eltze M, König H, Ullrich B, et al. Failure of AH11110A to functionally discriminate between alpha(1)-adrenoceptor subtypes A, B and D or between alpha(1)- and alpha(2)-adrenoceptors. Eur J Pharmacol, 2001, 415(2-3): 265-276.
- Macrocarpal I
Catalog No.:BCN1138
CAS No.:179388-54-6
- Macrocarpal H
Catalog No.:BCN1137
CAS No.:179388-53-5
- Sumanirole maleate
Catalog No.:BCC4112
CAS No.:179386-44-8
- 7-O-Methyleucomol
Catalog No.:BCN6830
CAS No.:17934-15-5
- Eucomol
Catalog No.:BCN6820
CAS No.:17934-12-2
- Bortezomib (PS-341)
Catalog No.:BCC1238
CAS No.:179324-69-7
- Sugetriol 6,9-diacetate
Catalog No.:BCN6960
CAS No.:17928-63-1
- Src I1
Catalog No.:BCC7733
CAS No.:179248-59-0
- Zearalenone
Catalog No.:BCC7831
CAS No.:17924-92-4
- Myricitrin
Catalog No.:BCN1136
CAS No.:17912-87-7
- N-Arachidonylglycine
Catalog No.:BCC7069
CAS No.:179113-91-8
- Curzerene
Catalog No.:BCN2352
CAS No.:17910-09-7
- SDZ 220-581 hydrochloride
Catalog No.:BCC4157
CAS No.:179411-93-9
- SDZ 220-581 Ammonium salt
Catalog No.:BCC1940
CAS No.:179411-94-0
- 2-Methoxyanofinic acid
Catalog No.:BCN7632
CAS No.:179457-70-6
- Leucodin
Catalog No.:BCN7105
CAS No.:17946-87-1
- PD 151746
Catalog No.:BCC5485
CAS No.:179461-52-0
- Caspofungin Acetate
Catalog No.:BCC4895
CAS No.:179463-17-3
- Terrestrosin D
Catalog No.:BCN2934
CAS No.:179464-23-4
- Prucalopride
Catalog No.:BCC5055
CAS No.:179474-81-8
- Prucalopride Succinat
Catalog No.:BCC4708
CAS No.:179474-85-2
- Rutacridone
Catalog No.:BCN7542
CAS No.:17948-33-3
- Venoterpine
Catalog No.:BCN3422
CAS No.:17948-42-4
- 1,2,3,4-Tetrahydronorharman-1-one
Catalog No.:BCN3690
CAS No.:17952-82-8
Competitive and allosteric interactions of 6-chloro-5,10-dihydro-5-[(1-methyl-4-piperidinyl)acetyl]-11H-di benzo[b,e][1, 4]diazepine-11-one hydrochloride (UH-AH 37) at muscarinic receptors, via distinct epitopes.[Pubmed:9890566]
Biochem Pharmacol. 1999 Jan 15;57(2):181-6.
6-Chloro-5,10-dihydro-5-[( 1-methyl-4-piperidinyl)acetyl]-11H-dibenzo[b,e][1,4]diazepine-11one++ + hydrochloride (UH-AH 37) is an analog of pirenzepine that has previously been reported to interact with classical muscarinic antagonists in a competitive manner, yet its binding has also been found to be sensitive to the same epitope as is that of the allosteric ligand gallamine. The present study was carried out with wild-type and chimeric muscarinic receptors to determine whether UH-AH 37 might also have an allosteric mode of action. In assays that detect only allosteric interactions, UH-AH 37 slowed the rate of dissociation of [3H]N-methylscopolamine (NMS) from all five muscarinic receptor subtypes, with the highest apparent affinity at m2. By contrast, studies carried out under equilibrium conditions have found UH-AH 37 to have the lowest affinity for the m2 subtype. Studies with m2/m5 chimeric receptors found the allosteric potency of UH-AH 37 to be sensitive to an epitope in the seventh transmembrane domain (TM). Again, this contrasts with equilibrium studies, wherein an epitope in the sixth TM has been implicated. Simultaneous analysis of the interactions between UH-AH 37 and [3H]NMS at the m2 receptor under equilibrium and non-equilibrium conditions found that a simple allosteric model could not accommodate both sets of data. On the other hand, the model did accommodate such data for gallamine; gallamine also displays concordance in order-of-potency and epitope sensitivity between equilibrium and non-equilibrium assays. Based on these results, we conclude that UH-AH 37 interacts at the classical muscarinic binding site with high affinity and at a second (allosteric) site with lower affinity.
Failure of AH11110A to functionally discriminate between alpha(1)-adrenoceptor subtypes A, B and D or between alpha(1)- and alpha(2)-adrenoceptors.[Pubmed:11275009]
Eur J Pharmacol. 2001 Mar;415(2-3):265-76.
The potency of the putatively alpha(1B)-adrenoceptor selective drug, 1-[biphenyl-2-yloxy]-4-imino-4-piperidin-1-yl-butan-2-ol (AH11110A), to antagonize contraction upon stimulation of alpha(1A)-adrenoceptors in rat vas deferens and rat perfused kidney, alpha(1B)-adrenoceptors in guinea-pig spleen, mouse spleen and rabbit aorta, and alpha(1D)-adrenoceptors in rat aorta and pulmonary artery was evaluated and compared to that of a number of subtype-discriminating antagonists. N-[3-[4-(2-Methoxyphenyl)-1-piperazinyl]propyl]-3-methyl-4-oxo-2-phenyl-4H-1-benz opyran-8-carboxamide (Rec 15/2739) and (+/-)-1,3,5-trimethyl-6-[[3-[4-((2,3-dihydro-2-hydroxymethyl)-1,4-benzodioxin-5-y l)-1-piperazinyl]propyl]amino]-2,4(1H,3H)-pyrimidinedione (B8805-033) were confirmed as selective for alpha(1A)-adrenoceptors, 8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4.5]decane-7,9-dione (BMY 7378), 8-[2-(1,4-benzodioxan-2-ylmethylamino)ethyl]-8-azaspiro[4.5]decane-7,9-dione (MDL 73005EF), and cystazosin were found to be selective for alpha(1D)-adrenoceptors, whereas spiperone was weakly selective for alpha(1B)-over alpha(1A)-adrenoceptors. However, from the functional affinity profile obtained for AH11110A at alpha(1A)-adrenoceptors (pA(2)=6.41 in rat vas deferens), alpha(1B)-adrenoceptors (pA(2)=5.40-6.54) and alpha(1D)-adrenoceptors (pA(2)=5.47-5.48), the affinity and presumed selectivity previously obtained for AH11110A in radioligand binding studies at native alpha(1B)- and cloned alpha(1b)-adrenoceptors (pK(i)=7.10-7.73) could not be confirmed. Additionally, AH11110A enhanced the general contractility of rat vas deferens, produced a bell-shaped dose-response curve of vasodilation in perfused rat kidney, and its antagonism in most other tissues was not simply competitive. The affinity of AH11110A for prejunctional alpha(2)-adrenoceptors in rabbit vas deferens (pA(2)=5.44) was not much lower than that displayed for alpha(1)-adrenoceptor subtypes, revealing that AH11110A, besides alpha(1)-adrenoceptors, also interacts with alpha(2)-adrenoceptors, and thus may be unsuitable for alpha-adrenoceptor subtype characterization, at least in smooth muscle containing functional studies.


