PD 151746calpain inhibitor, cell-permeable CAS# 179461-52-0 |
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Calpeptin
Catalog No.:BCC2351
CAS No.:117591-20-5
- Acetyl-Calpastatin (184-210) (human)
Catalog No.:BCC2350
CAS No.:123714-50-1
- Calpain Inhibitor II, ALLM
Catalog No.:BCC1234
CAS No.:136632-32-1
- MDL 28170
Catalog No.:BCC2352
CAS No.:88191-84-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 179461-52-0 | SDF | Download SDF |
| PubChem ID | 5353866 | Appearance | Powder |
| Formula | C11H8FNO2S | M.Wt | 237.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 100 mg/mL (421.50 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
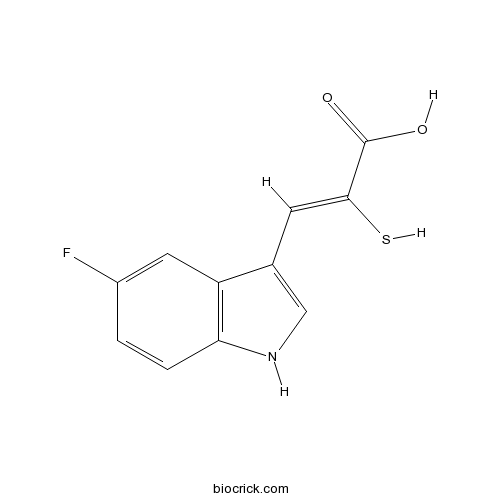
| Chemical Name | (Z)-3-(5-fluoro-1H-indol-3-yl)-2-sulfanylprop-2-enoic acid | ||
| SMILES | C1=CC2=C(C=C1F)C(=CN2)C=C(C(=O)O)S | ||
| Standard InChIKey | HWMQHECFXSVZGN-KMKOMSMNSA-N | ||
| Standard InChI | InChI=1S/C11H8FNO2S/c12-7-1-2-9-8(4-7)6(5-13-9)3-10(16)11(14)15/h1-5,13,16H,(H,14,15)/b10-3- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | PD151746 is a calpain inhibitor, shows a 20-fold selectivity for u-calpain (Ki = 0.26 ± 0.03 μM) over m-calpain (Ki = 5.33 ± 0.77 μM).
IC50 value: 0.26 ± 0.03 μM (Ki, for μ-calpain), 5.33 ± 0.77 μM (Ki, for m-calpain) [1]
Target: calpain
in vitro: The μ-calpain inhibitor PD 151746 decreases oxLDL-induced cytotoxicity. [2] References: | |||||

PD 151746 Dilution Calculator

PD 151746 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.215 mL | 21.0748 mL | 42.1496 mL | 84.2993 mL | 105.3741 mL |
| 5 mM | 0.843 mL | 4.215 mL | 8.4299 mL | 16.8599 mL | 21.0748 mL |
| 10 mM | 0.4215 mL | 2.1075 mL | 4.215 mL | 8.4299 mL | 10.5374 mL |
| 50 mM | 0.0843 mL | 0.4215 mL | 0.843 mL | 1.686 mL | 2.1075 mL |
| 100 mM | 0.0421 mL | 0.2107 mL | 0.4215 mL | 0.843 mL | 1.0537 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
PD 151746 is a potent and selective inhibitor of calpain with Ki value of 0.26 μM for μ-Calpain.
Calpain is a calcium-dependent, non-lysosomal cysteine protease that expressed in mammals and many other organisms. Calpain plays an important role in cell mobility and cell cycle progression.
PD 151746 is a cell-permeable, potent and selective calpain inhibitor. PD151746 significantly inhibited NMDA-induced α-spectrin breakdown product (SBDP) of 145 kDa and completely inhibited the fragmentation of calmodulin-dependent protein kinase II-α (CaMPK-IIα) and nitric oxide synthase (nNOS), which were cleaved by calpain [1]. In cerebellar granule cells, PD151746 inhibited serum/potassium (S/K) withdrawal induced apoptosis by 29% through inhibition of calpain. Also, PD151746 inhibited the increase of MEF2 phosphorylation and cdk5/p25 formation and inhibited caspase-3 activity [2]. In human hepatoma G2 cells, PD151746 significantly reduced insulin-stimulated glycogen synthesis and increased the amount of protein tyrosine phosphatase-ε (PTPε), which suggested that calpain played an important role in regulation of insulin-stimulated glycogen synthesis [3]. In HEK-293 cells expressing human formyl peptide receptor (hFPR) or hFPR-like 1 (hFPRL1), PD151746 increased cytoplasmic free Ca2+ ([Ca2+]i) [4].
References:
[1]. Hajimohammadreza I, Raser KJ, Nath R, et al. Neuronal nitric oxide synthase and calmodulin-dependent protein kinase IIalpha undergo neurotoxin-induced proteolysis. J Neurochem, 1997, 69(3): 1006-1013.
[2]. Verdaguer E, Alvira D, Jiménez A, et al. Inhibition of the cdk5/MEF2 pathway is involved in the antiapoptotic properties of calpain inhibitors in cerebellar neurons. Br J Pharmacol, 2005, 145(8): 1103-1011.
[3]. Meier M, Klein HH, Kramer J, et al. Calpain inhibition impairs glycogen syntheses in HepG2 hepatoma cells without altering insulin signaling. J Endocrinol, 2007, 193(1): 45-51.
[4]. Fujita H, Kato T, Watanabe N, et al. Stimulation of human formyl peptide receptors by calpain inhibitors: homology modeling of receptors and ligand docking simulation. Arch Biochem Biophys, 2011, 516(2): 121-127.
- Leucodin
Catalog No.:BCN7105
CAS No.:17946-87-1
- 2-Methoxyanofinic acid
Catalog No.:BCN7632
CAS No.:179457-70-6
- SDZ 220-581 Ammonium salt
Catalog No.:BCC1940
CAS No.:179411-94-0
- SDZ 220-581 hydrochloride
Catalog No.:BCC4157
CAS No.:179411-93-9
- AH 11110 hydrochloride
Catalog No.:BCC6883
CAS No.:179388-65-9
- Macrocarpal I
Catalog No.:BCN1138
CAS No.:179388-54-6
- Macrocarpal H
Catalog No.:BCN1137
CAS No.:179388-53-5
- Sumanirole maleate
Catalog No.:BCC4112
CAS No.:179386-44-8
- 7-O-Methyleucomol
Catalog No.:BCN6830
CAS No.:17934-15-5
- Eucomol
Catalog No.:BCN6820
CAS No.:17934-12-2
- Bortezomib (PS-341)
Catalog No.:BCC1238
CAS No.:179324-69-7
- Sugetriol 6,9-diacetate
Catalog No.:BCN6960
CAS No.:17928-63-1
- Caspofungin Acetate
Catalog No.:BCC4895
CAS No.:179463-17-3
- Terrestrosin D
Catalog No.:BCN2934
CAS No.:179464-23-4
- Prucalopride
Catalog No.:BCC5055
CAS No.:179474-81-8
- Prucalopride Succinat
Catalog No.:BCC4708
CAS No.:179474-85-2
- Rutacridone
Catalog No.:BCN7542
CAS No.:17948-33-3
- Venoterpine
Catalog No.:BCN3422
CAS No.:17948-42-4
- 1,2,3,4-Tetrahydronorharman-1-one
Catalog No.:BCN3690
CAS No.:17952-82-8
- PD 150606
Catalog No.:BCC2353
CAS No.:179528-45-1
- Cynaustraline
Catalog No.:BCN2048
CAS No.:17958-37-1
- Cynaustine
Catalog No.:BCN1951
CAS No.:17958-39-3
- Amabiline
Catalog No.:BCN1950
CAS No.:17958-43-9
- Macrocarpal J
Catalog No.:BCN1139
CAS No.:179603-47-5
Co-expression of AFAP1-AS1 and PD-1 predicts poor prognosis in nasopharyngeal carcinoma.[Pubmed:28380458]
Oncotarget. 2017 Jun 13;8(24):39001-39011.
Nasopharyngeal carcinoma (NPC) carries a high potential for metastasis and immune escape, with a great risk of relapse after primary treatment. Through analysis of whole genome expression profiling data in NPC samples, we found that the expression of a long non-coding RNA (lncRNA), actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1), is significantly correlated with the immune escape marker programmed death 1 (PD-1). We therefore assessed the expression of AFAP1-AS1 and PD-1 in a cohort of 96 paraffin-embedded NPC samples and confirmed that AFAP1-AS1 and PD-1 are co-expressed in infiltrating lymphocytes in NPC tissue. Moreover, patients with high expression of AFAP1-AS1 or PD-1 in infiltrating lymphocytes were more prone to distant metastasis, and NPC patients with positive expression of both AFAP1-AS1 and PD-1 had the poorest prognosis. This study suggests that AFAP1-AS1 and PD-1 may be potential therapeutic targets in NPC and that patients with co-expression of AFAP1-AS1 and PD-1 may be ideal candidates for future clinical trials of anti-PD-1 immune therapy.
Mechanistic insight into the regioselectivity of Pd(ii)-catalyzed C-H functionalization of N-methoxy cinnamamide.[Pubmed:28379232]
Dalton Trans. 2017 Apr 19;46(16):5288-5296.
Computational studies have been applied to gain insight into the mechanism of Pd(ii) catalyzed alpha-C-H functionalization of N-methoxy cinnamamide. The results show that the whole catalytic cycle proceeds via sequential six steps, including (i) catalyst Pd(t-BuNC)2 oxidation with O2, (ii) O-H deprotonation, (iii) t-BuNC migratory insertion to the Pd-C bond, (iv) acyl migration, (v) C-H activation and (vi) reductive elimination. The regioselectivity for different C-H activation sites depends on the coordination structures of alpha-C or beta-C to the palladium(ii) center. The coordination of alpha-C to the palladium(ii) center shows a regular planar quadrilateral structure, which is stable. However, the beta-C coordinating to the palladium(ii) center mainly exhibits a distorted quadrilateral structure, which is relatively unstable. Thus, the barrier of alpha-C-H activation is much lower than that of beta-C-H activation. The present results provide a deep understanding of the site-selectivity of C-H activation.
Three 1D cyanide-bridged M(Ni, Pd, Pt)-Mn(II) Coordination Polymer: Synthesis, Crystal Structure and Magnetic Properties.[Pubmed:28380238]
Acta Chim Slov. 2017 Mac;64(1):215-220.
Three tetracyanide-containing building blocks K2[M(CN)4] (M = Ni, Pd, Pt) and one semi-closed macrocycle seven-coordinated manganese(II) compound have been employed to assemble cyanide-bridged heterometallic complexes, resulting in three cyanide-bridged MII-MnII complexes: [Mn(L)][Ni(CN)4] . 2H2O (1) [Mn(L)][Pd(CN)4] (2) and [Mn(L)][Pt(CN)4] (3) (L = 2,6-bis[1-(2-(N-methylamino)ethylimino)ethyl]pyridine). Single-crystal X-ray diffraction analysis shows their similar one-dimensional structure consisting of the alternating [Mn(L)]2+ species and [M(CN)4]2- building blocks, generating a cyanide-bridged neutral polymeric chain. In all three isostructural complexes the coordination geometry of manganese ion is a slightly distorted pentagonal-bipyramidal with the two cyanide nitrogen atoms at the trans positions and N5 coordinating mode at the equatorial plane from ligand L. Investigation over magnetic properties of these complexes reveals very weak antiferromagnetic interaction between neighboring Mn(II) ions bridged by the long NC-M-CN unit. A best-fit to the magnetic susceptibility of complexes 1-3 leads to the magnetic coupling constant of J = -0.081, -0.103 and -0.14 cm-1, respectively.
Pd-Catalyzed Autotandem Reactions with N-Tosylhydrazones. Synthesis of Condensed Carbo- and Heterocycles by Formation of a C-C Single Bond and a C horizontal lineC Double Bond on the Same Carbon Atom.[Pubmed:28379703]
Org Lett. 2017 Apr 21;19(8):2034-2037.
A new Pd-catalyzed autotandem reaction is introduced that consists of the cross-coupling of a benzyl bromide with a N-tosylhydrazone followed by an intramolecular Heck reaction with an aryl bromide. During the process, a single and a double C-C bond are formed on the same carbon atom. Two different arrangements for the reactive functional groups are possible, rendering great flexibility to the transformation. The same strategy led to 9-methylene-9H-fluorenes, 9-methylene-9H-xanthenes, 9-methylene-9,10-dihydroacridines, and also dihydropyrroloisoquinoline and dihydroindoloisoquinoline derivatives.


