N-ArachidonylglycineEndogenous GlyT2 inhibitor CAS# 179113-91-8 |
- Phellodendrine chloride
Catalog No.:BCN5934
CAS No.:104112-82-5
- Lobetyolin
Catalog No.:BCN5894
CAS No.:136085-37-5
- Neferine
Catalog No.:BCN6338
CAS No.:2292-16-2
- Liensinine
Catalog No.:BCN6337
CAS No.:2586-96-1
- Orientin
Catalog No.:BCN4984
CAS No.:28608-75-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

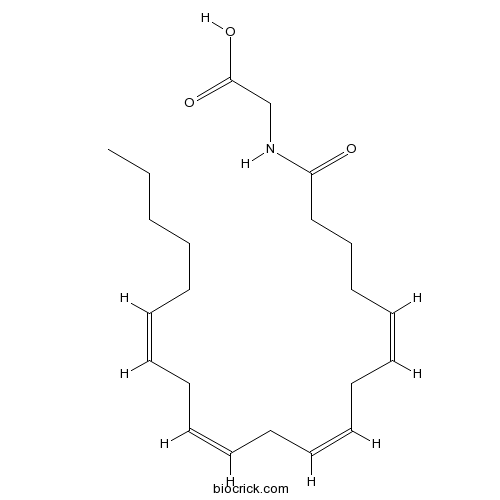
| Cas No. | 179113-91-8 | SDF | Download SDF |
| PubChem ID | 5283389 | Appearance | Powder |
| Formula | C22H35NO3 | M.Wt | 361.52 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | NAGly | ||
| Solubility | Soluble to 100 mM in ethanol | ||
| Chemical Name | 2-[[(5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoyl]amino]acetic acid | ||
| SMILES | CCCCCC=CCC=CCC=CCC=CCCCC(=O)NCC(=O)O | ||
| Standard InChIKey | YLEARPUNMCCKMP-DOFZRALJSA-N | ||
| Standard InChI | InChI=1S/C22H35NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21(24)23-20-22(25)26/h6-7,9-10,12-13,15-16H,2-5,8,11,14,17-20H2,1H3,(H,23,24)(H,25,26)/b7-6-,10-9-,13-12-,16-15- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Endogenous anandamide-like compound. Lacks affinity for CB1 receptors (Ki > 10 μM), VR1 receptors (EC50 > 10 μM) and anandamide transporters (IC50 > 50 μM) but causes hot-plate analgesia in mice when given orally, and suppresses tonic inflammatory pain. Also endogenous GlyT2 inhibitor. |

N-Arachidonylglycine Dilution Calculator

N-Arachidonylglycine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7661 mL | 13.8305 mL | 27.661 mL | 55.322 mL | 69.1525 mL |
| 5 mM | 0.5532 mL | 2.7661 mL | 5.5322 mL | 11.0644 mL | 13.8305 mL |
| 10 mM | 0.2766 mL | 1.383 mL | 2.7661 mL | 5.5322 mL | 6.9152 mL |
| 50 mM | 0.0553 mL | 0.2766 mL | 0.5532 mL | 1.1064 mL | 1.383 mL |
| 100 mM | 0.0277 mL | 0.1383 mL | 0.2766 mL | 0.5532 mL | 0.6915 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Curzerene
Catalog No.:BCN2352
CAS No.:17910-09-7
- H-Asp(OtBu)-OtBu.HCl
Catalog No.:BCC2893
CAS No.:1791-13-5
- PHCCC
Catalog No.:BCC6895
CAS No.:179068-02-1
- CPCCOEt
Catalog No.:BCC6896
CAS No.:179067-99-3
- FT-207 (NSC 148958)
Catalog No.:BCC4455
CAS No.:17902-23-7
- 3-(4-Pyridyl)-Alanine
Catalog No.:BCC2651
CAS No.:178933-04-5
- NGD 94-1
Catalog No.:BCC7636
CAS No.:178928-68-2
- Salviaflaside
Catalog No.:BCN8330
CAS No.:178895-25-5
- Myricadiol
Catalog No.:BCN1135
CAS No.:17884-88-7
- Vitexolide D
Catalog No.:BCN6739
CAS No.:1788090-69-6
- SNC 162
Catalog No.:BCC7103
CAS No.:178803-51-5
- 12-Hydroxyisobakuchiol
Catalog No.:BCN3609
CAS No.:178765-55-4
- Myricitrin
Catalog No.:BCN1136
CAS No.:17912-87-7
- Zearalenone
Catalog No.:BCC7831
CAS No.:17924-92-4
- Src I1
Catalog No.:BCC7733
CAS No.:179248-59-0
- Sugetriol 6,9-diacetate
Catalog No.:BCN6960
CAS No.:17928-63-1
- Bortezomib (PS-341)
Catalog No.:BCC1238
CAS No.:179324-69-7
- Eucomol
Catalog No.:BCN6820
CAS No.:17934-12-2
- 7-O-Methyleucomol
Catalog No.:BCN6830
CAS No.:17934-15-5
- Sumanirole maleate
Catalog No.:BCC4112
CAS No.:179386-44-8
- Macrocarpal H
Catalog No.:BCN1137
CAS No.:179388-53-5
- Macrocarpal I
Catalog No.:BCN1138
CAS No.:179388-54-6
- AH 11110 hydrochloride
Catalog No.:BCC6883
CAS No.:179388-65-9
- SDZ 220-581 hydrochloride
Catalog No.:BCC4157
CAS No.:179411-93-9
N-arachidonylglycine causes ROS production and cytochrome c release in liver mitochondria.[Pubmed:19501648]
Free Radic Biol Med. 2009 Sep 1;47(5):585-92.
N-Arachidonylglycine (NA-Gly) is an amino acid derivative of arachidonic acid. This compound is structurally related to anandamide (arachidonylethanolamine), which is considered an endogenous ligand of the cannabinoid receptor. NA-Gly is present at relatively high levels in the spinal cord, small intestine, and kidneys and at lower, but remarkable, levels in testes, lungs, and liver. The presence of varying levels in different organs suggests multiple functions in addition to the reported anti-inflammatory and pain suppression actions. Here a study on the interaction of NA-Gly with isolated mitochondria is reported. The results show that micromolar concentrations of NA-Gly cause: (i) an increase in the resting state respiration with both glutamate plus malate and succinate as substrates and (ii) a decrease in either ADP- or uncoupler-activated respiration. Whereas the stimulated resting state respiration was substantially reduced by cyclosporin A (CsA), the NA-Gly-inhibited State 3 respiration was almost unaffected. Measurements by blot analysis showed that NA-Gly caused a CsA-sensitive cytochrome c release. Under these conditions no matrix swelling could be detected. Experiments are also presented showing that NA-Gly caused a respiration-dependent large ROS production, which seems in turn to be responsible for the inhibition of electron transport activity and cytochrome c release.
Extracellular loops 2 and 4 of GLYT2 are required for N-arachidonylglycine inhibition of glycine transport.[Pubmed:19875446]
J Biol Chem. 2009 Dec 25;284(52):36424-30.
Concentrations of extracellular glycine in the central nervous system are regulated by Na(+)/Cl(-)-dependent glycine transporters, GLYT1 and GLYT2. N-Arachidonylglycine (NAGly) is an endogenous inhibitor of GLYT2 with little or no effect on GLYT1 and is analgesic in rat models of neuropathic and inflammatory pain. Understanding the molecular basis of NAGly interactions with GLYT2 may allow for the development of novel therapeutics. In this study, chimeric transporters were used to determine the structural basis for differences in NAGly sensitivity between GLYT1 and GLYT2 and also the actions of a series of related N-arachidonyl amino acids. Extracellular loops 2 and 4 of GLYT2 are important in the selective inhibition of GLYT2 by NAGly and by the related compounds N-arachidonyl-gamma-aminobutyric acid and N-arachidonyl-d-alanine, whereas only the extracellular loop 4 of GLYT2 is required for N-arachidonyl-l-alanine inhibition of transport. These observations suggest that the structure of the head group of these compounds is important in determining how they interact with extracellular loops 2 and 4 of GLYT2. Site-directed mutagenesis of GLYT2 EL4 residues was used to identify the key residues Arg(531), Lys(532), and Ile(545) that contribute to the differences in NAGly sensitivity.
Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92.[Pubmed:18499677]
J Biol Chem. 2008 Jul 25;283(30):21054-64.
A series of small compounds acting at the orphan G protein-coupled receptor GPR92 were screened using a signaling pathway-specific reporter assay system. Lipid-derived molecules including farnesyl pyrophosphate (FPP), N-Arachidonylglycine (NAG), and lysophosphatidic acid were found to activate GPR92. FPP and lysophosphatidic acid were able to activate both G(q/11)- and G(s)-mediated signaling pathways, whereas NAG activated only the G(q/11)-mediated signaling pathway. Computer-simulated modeling combined with site-directed mutagenesis of GPR92 indicated that Thr(97), Gly(98), Phe(101), and Arg(267) of GPR92 are responsible for the interaction of GPR92 with FPP and NAG. Reverse transcription-PCR analysis revealed that GPR92 mRNA is highly expressed in the dorsal root ganglia (DRG) but faint in other brain regions. Peripheral tissues including, spleen, stomach, small intestine, and kidney also expressed GPR92 mRNA. Immunohistochemical analysis revealed that GPR92 is largely co-localized with TRPV1, a nonspecific cation channel that responds to noxious heat, in mouse and human DRG. FPP and NAG increased intracellular Ca(2+) levels in cultured DRG neurons. These results suggest that FPP and NAG play a role in the sensory nervous system through activation of GPR92.
Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18.[Pubmed:16844083]
Biochem Biophys Res Commun. 2006 Sep 1;347(3):827-32.
An orphan G-protein-coupled receptor, GPR18, was cloned on the basis of degenerate-oligonucleotide PCR analysis of HUT 102 cells using primers designed from the conservative regions of the human chemokine receptor. GPR18 was expressed significantly in lymphoid cell lines, but not in non-lymphoid hematopoietic cell lines. Moreover, the expression of the GPR18 gene was higher in peripheral lymphocyte subsets (CD4(+), CD4(+)CD45RA(+), CD4(+)CD45RO(+), CD8(+), and CD19(+)) than in monocytes and lymphoid cell lines, and was increased after stimulation with phytohemagglutinin. By screening using a lipid library, N-Arachidonylglycine (NAGly) induced an increase in intracellular Ca(2+) concentration in GPR18-transfected cells, which was significantly greater than that in mock-transfected cells. NAGly also inhibited forskolin-induced cAMP production in a pertussis toxin-sensitive manner in the GPR18-transfected CHO cells. This is the first study to demonstrate that NAGly is a natural ligand for GPR18.
Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain.[Pubmed:11518719]
J Biol Chem. 2001 Nov 16;276(46):42639-44.
In mammals, specific lipids and amino acids serve as crucial signaling molecules. In bacteria, conjugates of lipids and amino acids (referred to as lipoamino acids) have been identified and found to possess biological activity. Here, we report that mammals also produce lipoamino acids, specifically the arachidonyl amino acids. We show that the conjugate of arachidonic acid and glycine (N-Arachidonylglycine (NAGly)) is present in bovine and rat brain as well as other tissues and that it suppresses tonic inflammatory pain. The biosynthesis of NAGly and its degradation by the enzyme fatty acid amide hydrolase can be observed in rat brain tissue. In addition to NAGly, bovine brain produces at least two other arachidonyl amino acids: N-arachidonyl gamma-aminobutyric acid (NAGABA) and N-arachidonylalanine. Like NAGly, NAGABA inhibits pain. These findings open the door to the identification of other members of this new class of biomolecules, which may be integral to pain regulation and a variety of functions in mammals.
Oxidative metabolism of anandamide.[Pubmed:10785540]
Prostaglandins Other Lipid Mediat. 2000 Apr;61(1-2):29-41.
In addition to the well studied hydrolytic metabolism of anandamide, a number of oxidative processes are also possible. Several routes somewhat analogous to the metabolism of free arachidonic acid have been reported. These involve mediation by various lipoxygenases and COX-2 and lead to ethanolamide analogs of the prostaglandins and HETES. The physiological significance of these products is not well understood at this time. There are also preliminary data suggesting a pathway involving oxidation of the hydroxy group of anandamide to a putative metabolite, N-arachidonyl glycine (AA-gly). This molecule displays activities in experimental models that suggest that it may play a role in some of the activities attributed to its precursor, anandamide.
Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor.[Pubmed:9057852]
J Med Chem. 1997 Feb 28;40(5):659-67.
In order to establish the structural requirements for binding to the brain cannabinoid receptor (CB1), we have synthesized numerous fatty acid amides, ethanolamides, and some related simple derivatives and have determined their Ki values. A few alpha-methyl- or alpha, alpha-dimethylarachidonoylalkylamides were also examined. In the 20:4, n-6 series, the unsubstituted amide is inactive; N-monoalkylation, at least up to a branched pentyl group, leads to significant binding. N,N-Dialkylation, with or without hydroxylation on one of the alkyl groups, leads to elimination of activity. Hydroxylation of the N-monoalkyl group at the omega carbon atom retains activity. In the 20x, n-6 series, x has to be either 3 or 4; the presence of only two double bonds leads to inactivation. In the n-3 series, the limited data reported suggest that the derived ethanolamides are either inactive or less active than comparable compounds in the n-6 series. Alkylation or dialkylation of the alpha carbon adjacent to the carbonyl group retains the level of binding in the case of anandamide (compounds 48, 49); however, alpha-monomethylation or alpha,alpha-dimethylation of N-propyl derivatives (50-53) potentiates binding and leads to the most active compounds seen in the present work (Ki values of 6.9 +/- 0.7 to 8.4 +/- 1.1 nM). We have confirmed that the presence of a chiral center on the N-alkyl substituent may lead to enantiomers which differ in their levels of binding (compounds 54, 57 and 55, 56).


