8-pCPT-2-O-Me-cAMP-AMCAS# 1152197-23-3 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
- Levistilide A
Catalog No.:BCN1197
CAS No.:88182-33-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1152197-23-3 | SDF | Download SDF |
| PubChem ID | 90488978 | Appearance | Powder |
| Formula | C20H21ClN5O8PS | M.Wt | 557.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 007-AM | ||
| Solubility | Soluble to 100 mM in DMSO | ||
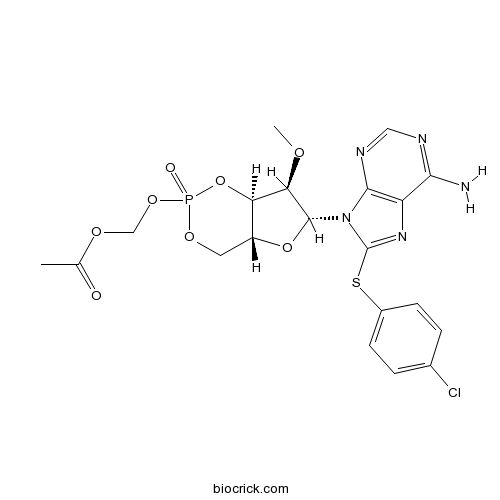
| Chemical Name | [(4aR,6R,7R,7aR)-6-[6-amino-8-(4-chlorophenyl)sulfanylpurin-9-yl]-7-methoxy-2-oxo-4a,6,7,7a-tetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-2-yl]oxymethyl acetate | ||
| SMILES | CC(=O)OCOP1(=O)OCC2C(O1)C(C(O2)N3C4=C(C(=NC=N4)N)N=C3SC5=CC=C(C=C5)Cl)OC | ||
| Standard InChIKey | FZMWUFYPEVDWPA-SILPBKOMSA-N | ||
| Standard InChI | InChI=1S/C20H21ClN5O8PS/c1-10(27)30-9-32-35(28)31-7-13-15(34-35)16(29-2)19(33-13)26-18-14(17(22)23-8-24-18)25-20(26)36-12-5-3-11(21)4-6-12/h3-6,8,13,15-16,19H,7,9H2,1-2H3,(H2,22,23,24)/t13-,15-,16-,19-,35?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective Epac activator; cAMP analog. Induces Rap activation and junction tightening in HUVECs; triggers adhesion of Jurkat-Epac1 cells to fibronectin. Stimulates insulin secretion in rat INS-1 cells. More potent, cell-permeable analog of 8CPT-2Me-cAMP.This product is a mixture of axial and equatorial isomers. Both isomers give 8CPT-2Me-cAMP after esterase cleavage. |

8-pCPT-2-O-Me-cAMP-AM Dilution Calculator

8-pCPT-2-O-Me-cAMP-AM Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7924 mL | 8.9622 mL | 17.9244 mL | 35.8487 mL | 44.8109 mL |
| 5 mM | 0.3585 mL | 1.7924 mL | 3.5849 mL | 7.1697 mL | 8.9622 mL |
| 10 mM | 0.1792 mL | 0.8962 mL | 1.7924 mL | 3.5849 mL | 4.4811 mL |
| 50 mM | 0.0358 mL | 0.1792 mL | 0.3585 mL | 0.717 mL | 0.8962 mL |
| 100 mM | 0.0179 mL | 0.0896 mL | 0.1792 mL | 0.3585 mL | 0.4481 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- Carmoxirole hydrochloride
Catalog No.:BCC7278
CAS No.:115092-85-8
- Soyacerebroside II
Catalog No.:BCN6029
CAS No.:115074-93-6
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- A 1120
Catalog No.:BCC7775
CAS No.:1152782-19-8
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Siguazodan
Catalog No.:BCC6954
CAS No.:115344-47-3
- Kanshone A
Catalog No.:BCN7279
CAS No.:115356-18-8
- 2,2-Dimethyl-6-phenylpyrano[3,4-b]pyran-8-one
Catalog No.:BCN7280
CAS No.:1153624-36-2
- Kanshone B
Catalog No.:BCN7700
CAS No.:115370-61-1
Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: studies with 8-pCPT-2'-O-Me-cAMP-AM.[Pubmed:19244230]
J Biol Chem. 2009 Apr 17;284(16):10728-36.
To ascertain the identities of cyclic nucleotide-binding proteins that mediate the insulin secretagogue action of cAMP, the possible contributions of the exchange protein directly activated by cAMP (Epac) and protein kinase A (PKA) were evaluated in a pancreatic beta cell line (rat INS-1 cells). Assays of Rap1 activation, CREB phosphorylation, and PKA-dependent gene expression were performed in combination with live cell imaging and high throughput screening of a fluorescence resonance energy transfer-based cAMP sensor (Epac1-camps) to validate the selectivity with which acetoxymethyl esters (AM-esters) of cAMP analogs preferentially activate Epac or PKA. Selective activation of Epac or PKA was achieved following exposure of INS-1 cells to 8-pCPT-2'-O-Me-cAMP-AM or Bt(2)cAMP-AM, respectively. Both cAMP analogs exerted dose-dependent and glucose metabolism-dependent actions to stimulate insulin secretion, and when each was co-administered with the other, a supra-additive effect was observed. Because 2.4-fold more insulin was secreted in response to a saturating concentration (10 microm) of Bt(2)cAMP-AM as compared with 8-pCPT-2'-O-Me-cAMP-AM, and because the action of Bt(2)cAMP-AM but not 8-pCPT-2'-O-Me-cAMP-AM was nearly abrogated by treatment with 3 microm of the PKA inhibitor H-89, it is concluded that for INS-1 cells, it is PKA that acts as the dominant cAMP-binding protein in support of insulin secretion. Unexpectedly, 10-100 microm of the non-AM-ester of 8-pCPT-2'-O-Me-cAMP failed to stimulate insulin secretion and was a weak activator of Rap1 in INS-1 cells. Moreover, 10 microm of the AM-ester of 8-pCPT-2'-O-Me-cAMP stimulated insulin secretion from mouse islets, whereas the non-AM-ester did not. Thus, the membrane permeability of 8-pCPT-2'-O-Me-cAMP in insulin-secreting cells is so low as to limit its biological activity. It is concluded that prior reports documenting the failure of 8-pCPT-2'-O-Me-cAMP to act in beta cells, or other cell types, need to be re-evaluated through the use of the AM-ester of this cAMP analog.
Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM.[Pubmed:21099281]
Islets. 2009 Nov-Dec;1(3):260-5.
Epac2 is a cAMP-regulated guanine nucleotide exchange factor (cAMP-GEF) that is proposed to mediate stimulatory actions of the second messenger cAMP on mouse islet insulin secretion. Here we have used methods of islet perifusion to demonstrate that the acetoxymethyl ester (AM-ester) of an Epac-selective cAMP analog (ESCA) penetrates into mouse islets and is capable of potentiating both first and second phases of glucose-stimulated insulin secretion (GSIS). When used at low concentrations (1-10 muM), 8-pCPT-2'-O-Me-cAMP-AM activates Rap1 GTPase but exhibits little or no ability to activate protein kinase A (PKA), as validated in assays of in vitro PKA activity (phosphorylation of Kemptide), Ser (133) CREB phosphorylation status, RIP1-CRE-Luc reporter gene activity, and PKA-dependent AKAR3 biosensor activation. Since quantitative PCR demonstrates Epac2 mRNA to be expressed at levels ca. 5.3-fold greater than that of Epac1, available evidence indicates that Epac2 does in fact mediate stimulatory actions of cAMP on mouse islet GSIS.
PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2'-O-Me-cAMP-AM in human islets of Langerhans.[Pubmed:20009023]
Am J Physiol Endocrinol Metab. 2010 Mar;298(3):E622-33.
Potential insulin secretagogue properties of an acetoxymethyl ester of a cAMP analog (8-pCPT-2'-O-Me-cAMP-AM) that activates the guanine nucleotide exchange factors Epac1 and Epac2 were assessed using isolated human islets of Langerhans. RT-QPCR demonstrated that the predominant variant of Epac expressed in human islets was Epac2, although Epac1 was detectable. Under conditions of islet perifusion, 8-pCPT-2'-O-Me-cAMP-AM (10 microM) potentiated first- and second-phase 10 mM glucose-stimulated insulin secretion (GSIS) while failing to influence insulin secretion measured in the presence of 3 mM glucose. The insulin secretagogue action of 8-pCPT-2'-O-Me-cAMP-AM was associated with depolarization and an increase of [Ca(2+)](i) that reflected both Ca(2+) influx and intracellular Ca(2+) mobilization in islet beta-cells. As expected for an Epac-selective cAMP analog, 8-pCPT-2'-O-Me-cAMP-AM (10 microM) failed to stimulate phosphorylation of PKA substrates CREB and Kemptide in human islets. Furthermore, 8-pCPT-2'-O-Me-cAMP-AM (10 microM) had no significant ability to activate AKAR3, a PKA-regulated biosensor expressed in human islet cells by viral transduction. Unexpectedly, treatment of human islets with an inhibitor of PKA activity (H-89) or treatment with a cAMP antagonist that blocks PKA activation (Rp-8-CPT-cAMPS) nearly abolished the action of 8-pCPT-2'-O-Me-cAMP-AM to potentiate GSIS. It is concluded that there exists a permissive role for PKA activity in support of human islet insulin secretion that is both glucose dependent and Epac regulated. This permissive action of PKA may be operative at the insulin secretory granule recruitment, priming, and/or postpriming steps of Ca(2+)-dependent exocytosis.
A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion.[Pubmed:23066090]
Mol Pharmacol. 2013 Jan;83(1):122-8.
Exchange protein directly activated by cAMP (EPAC) and cAMP-dependent protein kinase (PKA) are two intracellular receptors that mediate the effects of the prototypic second messenger cAMP. Identifying pharmacological probes for selectively modulating EPAC activity represents a significant unmet need within the research field. Herein, we report the identification and characterization of 3-(5-tert-butyl-isoxazol-3-yl)-2-[(3-chloro-phenyl)-hydrazono]-3-oxo-propionitril e (ESI-09), a novel noncyclic nucleotide EPAC antagonist that is capable of specifically blocking intracellular EPAC-mediated Rap1 activation and Akt phosphorylation, as well as EPAC-mediated insulin secretion in pancreatic beta cells. Using this novel EPAC-specific inhibitor, we have probed the functional roles of overexpression of EPAC1 in pancreatic cancer cells. Our studies show that EPAC1 plays an important role in pancreatic cancer cell migration and invasion, and thus represents a potential target for developing novel therapeutic strategies for pancreatic cancer.
Isoform-specific antagonists of exchange proteins directly activated by cAMP.[Pubmed:23091014]
Proc Natl Acad Sci U S A. 2012 Nov 6;109(45):18613-8.
The major physiological effects of cAMP in mammalian cells are transduced by two ubiquitously expressed intracellular cAMP receptors, protein kinase A (PKA) and exchange protein directly activated by cAMP (EPAC), as well as cyclic nucleotide-gated ion channels in certain tissues. Although a large number of PKA inhibitors are available, there are no reported EPAC-specific antagonists, despite extensive research efforts. Here we report the identification and characterization of noncyclic nucleotide EPAC antagonists that are exclusively specific for the EPAC2 isoform. These EAPC2-specific antagonists, designated as ESI-05 and ESI-07, inhibit Rap1 activation mediated by EAPC2, but not EPAC1, with high potency in vitro. Moreover, ESI-05 and ESI-07 are capable of suppressing the cAMP-mediated activation of EPAC2, but not EPAC1 and PKA, as monitored in living cells through the use of EPAC- and PKA-based FRET reporters, or by the use of Rap1-GTP pull-down assays. Deuterium exchange mass spectroscopy analysis further reveals that EPAC2-specific inhibitors exert their isoform selectivity through a unique mechanism by binding to a previously undescribed allosteric site: the interface of the two cAMP binding domains, which is not present in the EPAC1 isoform. Isoform-specific EPAC pharmacological probes are highly desired and will be valuable tools for dissecting the biological functions of EPAC proteins and their roles in various disease states.


