Z-Asp-OHCAS# 1152-61-0 |
- Fulvestrant
Catalog No.:BCC1081
CAS No.:129453-61-8
- Bazedoxifene
Catalog No.:BCC1411
CAS No.:198481-32-2
- Bazedoxifene acetate
Catalog No.:BCC1412
CAS No.:198481-33-3
- (E)-2-Decenoic acid
Catalog No.:BCC1292
CAS No.:334-49-6
- Toremifene
Catalog No.:BCC2010
CAS No.:89778-26-7
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 1152-61-0 | SDF | Download SDF |
| PubChem ID | 2723942 | Appearance | Powder |
| Formula | C12H13NO6 | M.Wt | 267.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | N-Carbobenzyloxy-L-aspartic acid | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-(phenylmethoxycarbonylamino)butanedioic acid | ||
| SMILES | C1=CC=C(C=C1)COC(=O)NC(CC(=O)O)C(=O)O | ||
| Standard InChIKey | XYXYXSKSTZAEJW-VIFPVBQESA-N | ||
| Standard InChI | InChI=1S/C12H13NO6/c14-10(15)6-9(11(16)17)13-12(18)19-7-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,13,18)(H,14,15)(H,16,17)/t9-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Z-Asp-OH Dilution Calculator

Z-Asp-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7425 mL | 18.7126 mL | 37.4251 mL | 74.8503 mL | 93.5629 mL |
| 5 mM | 0.7485 mL | 3.7425 mL | 7.485 mL | 14.9701 mL | 18.7126 mL |
| 10 mM | 0.3743 mL | 1.8713 mL | 3.7425 mL | 7.485 mL | 9.3563 mL |
| 50 mM | 0.0749 mL | 0.3743 mL | 0.7485 mL | 1.497 mL | 1.8713 mL |
| 100 mM | 0.0374 mL | 0.1871 mL | 0.3743 mL | 0.7485 mL | 0.9356 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
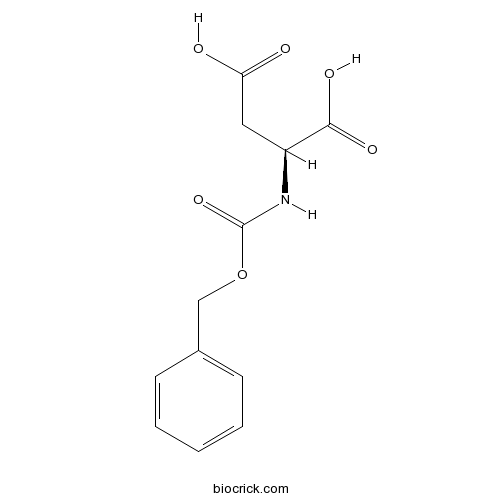
Z-Asp-OH
- ICI 199,441 hydrochloride
Catalog No.:BCC6792
CAS No.:115199-84-3
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- Carmoxirole hydrochloride
Catalog No.:BCC7278
CAS No.:115092-85-8
- Soyacerebroside II
Catalog No.:BCN6029
CAS No.:115074-93-6
- CNQX
Catalog No.:BCC6569
CAS No.:115066-14-3
- Desmethylxanthohumol
Catalog No.:BCN2997
CAS No.:115063-39-3
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
- 8-pCPT-2-O-Me-cAMP-AM
Catalog No.:BCC6305
CAS No.:1152197-23-3
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- A 1120
Catalog No.:BCC7775
CAS No.:1152782-19-8
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Siguazodan
Catalog No.:BCC6954
CAS No.:115344-47-3
- Kanshone A
Catalog No.:BCN7279
CAS No.:115356-18-8
Synthesis of an N-glucoasparagine analog as a building block for a V3-loop glycopeptide from gp120 of HIV-I.[Pubmed:9880906]
Carbohydr Res. 1998 Dec;313(2):107-16.
The preparative synthesis of a new N4-(2-acetamido-2-deoxy-beta-D-glucopyranosyl)-L-asparagine mimetic 1, starting from 2-amino-1,5-anhydro-2-deoxy-glucitol hydrochloride and Z-Asp-(OH)-OBn is described. This glycosyl-amino acid unit 1 is expected to show higher stabilities towards in vivo conditions. Further, the use of 1 as building block for the synthesis of modified glycopeptides using solid phase support is reported. The glycopeptide Ac-SXNTRKSIHIGPGRAF-NH2 having sugar-modified Asn2 mimics parts of the V3-loop structure containing the principle neutralizing determinant (PND) of HIV-1 and the naturally conserved glycosylation site within the V3 loop.
Influence of reaction conditions on syntheses of sweetener precursors catalyzed by thermolysin in tert-amyl alcohol.[Pubmed:9832308]
J Pept Res. 1998 Oct;52(4):300-4.
The activity of enzymes to form a peptide bond in organic solvent was greatly influenced by observed pH and water content. The precursors of two sweeteners, P-Asp-Xaa-OR (P=Z or For, Xaa-OR=Phe-OMe or Ala-OcHex), were synthesized by enzyme, and the reaction conditions were studied systematically. Z-Asp-OH was coupled with H-Phe-OMe or H-Ala-OcHex by thermolysin in tert-amyl alcohol. The best coupling results were obtained when the optimized observed pH was 8 or 9, and the water content was about 6% (V/V). The protecting group Z is better than For under the reaction conditions and H-Phe-OMe is a better nucleophile than H-Ala-OcHex. The expected optically pure peptides were obtained when the racemic amino acids were used as amino components in the starting materials. The physical constants of P-Asp-Xaa-OR synthesized by thermolysin are identical with those of peptides synthesized by chemical method.
Use of peak sharpening effects to improve the separation of chiral compounds with molecularly imprinted porous polymer layer open-tubular capillaries.[Pubmed:28211061]
Electrophoresis. 2017 Apr;38(8):1179-1187.
This investigation demonstrates the application of a new peak sharpening technique to improve the separation of difficult-to-resolve racemic mixtures in capillary electro-chromatography. Molecularly imprinted porous layer open tubular (MIP-PLOT) capillaries, prepared by a layer-on-layer polymerization approach with Z-l-Asp-OH as the template, were selected to validate the approach. SEM revealed that the polymer film thickness can be varied by changes in both the polymer composition and the layer-on-layer regime. Capillaries made with methacrylic acid as the functional monomer could not separate the Z-Asp-OH racemate, due to weak interactions between the MIP-PLOT material and the target analytes. In contrast, MIP-PLOT capillaries prepared with 4-vinylpyridine as the functional monomer resulted in increased ionic interactions with the target analytes. Separation of the enantiomers could be enhanced when a peak zone sharpening effect was exploited through the use of specific BGE compositions and by taking advantage of eigenpeak phenomena. In this manner, the position of a sharpening zone and the peak shape of the sample analytes could be fine-tuned, so that when the sharpening zone and the target analyte co-migrated the separation of the Z-l-Asp-OH enantiomer from its d-enantiomer in a racemic mixture could be achieved under overloading conditions.


