ICI 199,441 hydrochlorideCAS# 115199-84-3 |
- KY 02111
Catalog No.:BCC3628
CAS No.:1118807-13-8
- IWR-1-endo
Catalog No.:BCC5102
CAS No.:1127442-82-3
- CCT 031374 hydrobromide
Catalog No.:BCC6258
CAS No.:1219184-91-4
- XAV-939
Catalog No.:BCC1120
CAS No.:284028-89-3
- iCRT 14
Catalog No.:BCC5401
CAS No.:677331-12-3
- IWP-2
Catalog No.:BCC1665
CAS No.:686770-61-6
Quality Control & MSDS
Number of papers citing our products

Chemical structure

3D structure
| Cas No. | 115199-84-3 | SDF | Download SDF |
| PubChem ID | 3082717 | Appearance | Powder |
| Formula | C21H25Cl3N2O | M.Wt | 427.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
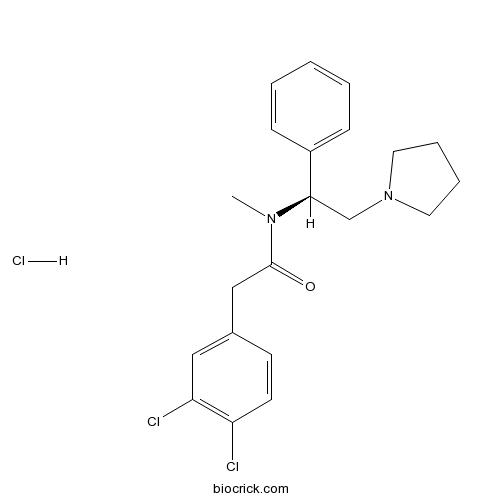
| Chemical Name | 2-(3,4-dichlorophenyl)-N-methyl-N-[(1S)-1-phenyl-2-pyrrolidin-1-ylethyl]acetamide;hydrochloride | ||
| SMILES | CN(C(CN1CCCC1)C2=CC=CC=C2)C(=O)CC3=CC(=C(C=C3)Cl)Cl.Cl | ||
| Standard InChIKey | VFLWVWZSDBTGQJ-VEIFNGETSA-N | ||
| Standard InChI | InChI=1S/C21H24Cl2N2O.ClH/c1-24(21(26)14-16-9-10-18(22)19(23)13-16)20(15-25-11-5-6-12-25)17-7-3-2-4-8-17;/h2-4,7-10,13,20H,5-6,11-12,14-15H2,1H3;1H/t20-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly potent κ agonist, 146-fold more active than U-50488 in vitro. |

ICI 199,441 hydrochloride Dilution Calculator

ICI 199,441 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3375 mL | 11.6877 mL | 23.3754 mL | 46.7508 mL | 58.4385 mL |
| 5 mM | 0.4675 mL | 2.3375 mL | 4.6751 mL | 9.3502 mL | 11.6877 mL |
| 10 mM | 0.2338 mL | 1.1688 mL | 2.3375 mL | 4.6751 mL | 5.8439 mL |
| 50 mM | 0.0468 mL | 0.2338 mL | 0.4675 mL | 0.935 mL | 1.1688 mL |
| 100 mM | 0.0234 mL | 0.1169 mL | 0.2338 mL | 0.4675 mL | 0.5844 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Caprarioside
Catalog No.:BCN7278
CAS No.:1151862-69-9
- 6-Epiharpagoside
Catalog No.:BCN3981
CAS No.:1151862-67-7
- Boc-D-Lys(Fmoc)-OH
Catalog No.:BCC3422
CAS No.:115186-31-7
- Antagonist G
Catalog No.:BCC5858
CAS No.:115150-59-9
- MK 571
Catalog No.:BCC7334
CAS No.:115104-28-4
- MK-571 sodium salt hydrate
Catalog No.:BCC8076
CAS No.:115103-85-0
- Tiagabine
Catalog No.:BCC5243
CAS No.:115103-54-3
- Carmoxirole hydrochloride
Catalog No.:BCC7278
CAS No.:115092-85-8
- Soyacerebroside II
Catalog No.:BCN6029
CAS No.:115074-93-6
- CNQX
Catalog No.:BCC6569
CAS No.:115066-14-3
- Desmethylxanthohumol
Catalog No.:BCN2997
CAS No.:115063-39-3
- 29-Norcycloart-23-ene-3,25-diol
Catalog No.:BCN4727
CAS No.:115040-04-5
- Z-Asp-OH
Catalog No.:BCC2793
CAS No.:1152-61-0
- Z-Met-OH
Catalog No.:BCC2760
CAS No.:1152-62-1
- 8-pCPT-2-O-Me-cAMP-AM
Catalog No.:BCC6305
CAS No.:1152197-23-3
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- A 1120
Catalog No.:BCC7775
CAS No.:1152782-19-8
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Siguazodan
Catalog No.:BCC6954
CAS No.:115344-47-3
Structure/activity studies related to 2-(3,4-dichlorophenyl)-N-methyl-N-[2-(1-pyrrolidinyl)-1-substituted- ethyl]acetamides: a novel series of potent and selective kappa-opioid agonists.[Pubmed:1659636]
J Med Chem. 1991 Nov;34(11):3149-58.
This paper describes the synthesis of a series of N-[2-(1-pyrrolidinyl)ethyl]acetamides 1, variously substituted at the carbon adjacent to the amide nitrogen (C1), and related analogues, together with their biological evaluation as opioid kappa agonists. In the first part of the study, the variants in N-acyl, N-alkyl, and amino functions were explored when the substituent at C1 was 1-methylethyl and the optimum was found to be exemplified by 2-(3,4-dichlorophenyl)-N-methyl-N-[(1S)-1-(1-methylethyl)-2- (1-pyrrolidinyl)ethyl]acetamide (13). Subsequently, racemic or chiral amino acids were used to introduce other alkyl and aryl substituents at C1 of the ethyl linking moiety. A series of potent compounds, bearing substituted-aryl groups at C1, were discovered, typified by 2-(3,4-dichloro-phenyl)-N-methyl-N-[(1R,S)-1-(3-aminophenyl)-2-(1- pyrrolidinyl)ethyl]acetamide (48), which was 5-fold more active as the racemate than 13 in vitro and exhibited potent naloxone-reversible analgesic effects (ED50 = 0.04 mg/kg sc) in a mouse abdominal constriction model.
2-(3,4-Dichlorophenyl)-N-methyl-N-[2-(1-pyrrolidinyl)-1-substituted- ethyl]-acetamides: the use of conformational analysis in the development of a novel series of potent opioid kappa agonists.[Pubmed:1846918]
J Med Chem. 1991 Jan;34(1):181-9.
This paper describes the synthesis of a series of N-[2-(1-pyrrolidinyl)ethyl]acetamides (1), methylated at C1 and/or C2 of the ethyl linking group, and their biological evaluation as opioid kappa agonists. Conformational analysis of corresponding desaryl analogues 2 suggested that only those compounds capable of occupying an energy minimum close to that of the known kappa agonist N-[2-(1-pyrrolidinyl)cyclohexyl] acetamide U-50488 might possess kappa agonist properties. Starting from chiral amino acids, other alkyl and aryl substituents were introduced at C1 of the ethyl-linking moiety, giving compounds capable of adopting the same conformation as U-50488. The most potent of these, 2-(3,4-dichlorophenyl)-N-methyl-N-[(1S)-1-phenyl-2-(1-pyrrolidinyl)ethyl] acetamide (8), was 146-fold more active than U-50488 in vitro in the mouse vas deferens model and exhibited potent naloxone-reversible analgesic effects (ED50 = 0.004 mg/kg sc) in an abdominal constriction model.


