(-)-Licarin BCAS# 51020-87-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 51020-87-2 | SDF | Download SDF |
| PubChem ID | 5384942 | Appearance | White powder |
| Formula | C20H20O4 | M.Wt | 324.37 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
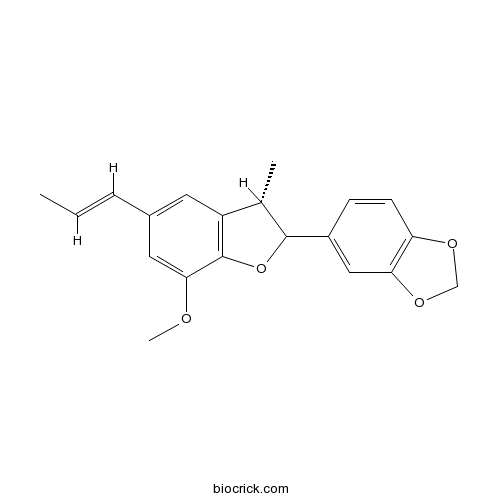
| Chemical Name | 5-[(3S)-7-methoxy-3-methyl-5-[(E)-prop-1-enyl]-2,3-dihydro-1-benzofuran-2-yl]-1,3-benzodioxole | ||
| SMILES | CC=CC1=CC2=C(C(=C1)OC)OC(C2C)C3=CC4=C(C=C3)OCO4 | ||
| Standard InChIKey | DMMQXURQRMNSBM-SWDBHPRBSA-N | ||
| Standard InChI | InChI=1S/C20H20O4/c1-4-5-13-8-15-12(2)19(24-20(15)18(9-13)21-3)14-6-7-16-17(10-14)23-11-22-16/h4-10,12,19H,11H2,1-3H3/b5-4+/t12-,19?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Licarin B can improve insulin sensitivity via PPARγ and activation of GLUT4 in the IRS-1/PI3K/AKT pathway in 3T3-L1 adipocytes, it as a promising bioactive for insulin resistance and associated complications through its partial PPARγ activity. |
| Targets | PPAR | GLUT | PI3K | Akt | IRS-1 |
| In vitro | Licarin B from Myristica fragrans improves insulin sensitivity via PPARγ and activation of GLUT4 in IRS-1/PI3K/AKT pathway in 3T3- L1 adipocytes[Reference: WebLink]Rsc Adv., 2016, 6(83):79859-70.Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors regulating lipid and glucose metabolism. |

(-)-Licarin B Dilution Calculator

(-)-Licarin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0829 mL | 15.4145 mL | 30.829 mL | 61.658 mL | 77.0725 mL |
| 5 mM | 0.6166 mL | 3.0829 mL | 6.1658 mL | 12.3316 mL | 15.4145 mL |
| 10 mM | 0.3083 mL | 1.5414 mL | 3.0829 mL | 6.1658 mL | 7.7072 mL |
| 50 mM | 0.0617 mL | 0.3083 mL | 0.6166 mL | 1.2332 mL | 1.5414 mL |
| 100 mM | 0.0308 mL | 0.1541 mL | 0.3083 mL | 0.6166 mL | 0.7707 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- (+)-Licarin A
Catalog No.:BCC9008
CAS No.:51020-86-1
- Demethylcephalotaxinone
Catalog No.:BCN7070
CAS No.:51020-45-2
- Isocorynoxeine
Catalog No.:BCN5003
CAS No.:51014-29-0
- 6''-O-Malonylgenistin
Catalog No.:BCN2772
CAS No.:51011-05-3
- Minecoside
Catalog No.:BCN5627
CAS No.:51005-44-8
- Galanthaminone
Catalog No.:BCN2867
CAS No.:510-77-0
- AMI-193
Catalog No.:BCC6679
CAS No.:510-74-7
- Echinocystic acid
Catalog No.:BCN5628
CAS No.:510-30-5
- Belladonnine
Catalog No.:BCN1892
CAS No.:510-25-8
- Voacangine
Catalog No.:BCN3224
CAS No.:510-22-5
- Trachelanthine
Catalog No.:BCN2042
CAS No.:510-19-0
- Tetramethylammonium
Catalog No.:BCN1816
CAS No.:51-92-3
- Salbutamol Sulfate
Catalog No.:BCC4338
CAS No.:51022-70-9
- Acipimox
Catalog No.:BCC4884
CAS No.:51037-30-0
- Flurbiprofen
Catalog No.:BCC3781
CAS No.:5104-49-4
- Z-GABA-OH,Z-gama-Abu-OH
Catalog No.:BCC2644
CAS No.:5105-78-2
- alpha-Lipomycin
Catalog No.:BCN1842
CAS No.:51053-40-8
- Wogonoside
Catalog No.:BCN1200
CAS No.:51059-44-0
- ATC 0065
Catalog No.:BCC7666
CAS No.:510732-84-0
- ATC 0175 hydrochloride
Catalog No.:BCC7657
CAS No.:510733-97-8
- Boc-Lys(Z)-pNA
Catalog No.:BCC3419
CAS No.:51078-31-0
- Pyrrolidinedithiocarbamate ammonium
Catalog No.:BCC6766
CAS No.:5108-96-3
- Methyl p-hydroxyphenyllactate
Catalog No.:BCN6669
CAS No.:51095-47-7
- 2,7-Dihydrohomoerysotrine
Catalog No.:BCN5629
CAS No.:51095-85-3
Matrix Solid-Phase Dispersion Combined with HPLC-DAD for Simultaneous Determination of Nine Lignans in Saururus chinensis.[Pubmed:30272133]
J Chromatogr Sci. 2019 Feb 1;57(2):186-193.
A simple and rapid method, based on matrix solid-phase dispersion (MSPD) and high-performance liquid chromatography (HPLC) was developed for simultaneous determination of nine lignans, including (-)-(7R,8R)-machilin D (Wang, C., Wang, P., Chen, X., Wang, W., Jin, Y.; Saururus chinensis (Lour.) Baill blocks enterovirus 71 infection by hijacking MEK1-ERK signaling pathway; Antiviral Research, (2015); 119:47-56), dihydroguaiaretic acid (Quan, Z., Lee, Y.J., Yang, J.H., Lu,Y., Li,Y., Lee,Y.K., et al.; Ethanol extracts of Saururus chinensis suppress ovalbumin-sensitization airway inflammation; Journal of Ethnopharmacology, (2010); 132:143-149.), sauchinone (Zhuang, T., Liang, J.Y., Sun, J.B., Wu, Y., Huang, L.R., Qu, W.; Secondary metabolites from Saururus chinensis and their chemotaxonomic significance; Biochemical Systematics and Ecology, (2014); 56:95-98.), rel-(7S,8S,7'R,8'R)-3,3',4,4',5,5'-hexamethoxy-7.O.7',8.8'-lignan (Tsai, W.J., Shen, C.C., Tsai, T.H., Lin, L.C.; Lignans from the aerial parts of Saururus chinensis: isolation, structural characterization, and their effects on platelet aggregation; Journal of Natural Products, (2014); 77:125-131), licarin A (Cui, H., Xu, B., Wu, T., Xu, J., Yuan, Y., Gu, Q.; Potential antiviral lignans from the roots of Saururus chinensis with activity against Epstein-Barr virus lytic replication; Journal of Natural Products, (2014); 77:100-110.), manassantin A (Lu, Y., Piao, D., Zhang, H., Li, X., Chao, G.H., Park, S.J., et al.; Saucerneol F inhibits tumor necrosis factor-alpha and IL-6 production by suppressing Fyn-mediated pathways in FcepsilonRI-mediated mast cells; Food and Chemical Toxicology, (2013); 59:696-702.), saurucinol I (Kwon, O.E., Lee, H.S., Lee, S.W., Chung, M.Y., Bae, K.H., Rho, M.C., et al.; Manassantin A and B isolated from Saururus chinensis inhibit TNF-alpha-induced cell adhesion molecule expression of human umbilical vein endothelial cells; Archives of Pharmacal Research, (2005); 28:55-60.), manassantin B (Hwang, B.Y., Lee, J.H., Jung, H.S., Kim, K.S., Nam, J.B., Hong, Y.S., et al.; Sauchinone, a lignan from Saururus chinensis, suppresses iNOS expression through the inhibition of transactivation activity of RelA of NF-kappaB; Planta Medica, (2003); 69:1096-01.) and (-)-Licarin B (Hwang, B.Y., Lee, J.H., Nam, J.B., Hong, Y.S., Lee, J.J.; Lignans from Saururus chinensis inhibiting the transcription factor NF-kappaB; Phytochemistry, (2003); 64:765-771.) in Saururus chinensis. The parameters of MSPD were optimized to be that 0.2 g of sample, blended with 0.4 g silica gel, and eluted with 5 mL of methanol. The separation was carried out on a C18 column with acidified aqueous acetonitrile gradients. The established method was fully validated in terms of linearity (r2 >/= 0.9994), sensitivity, precision (RSD
Phytochemical investigation on Myristica fragrans stem bark.[Pubmed:29607669]
Nat Prod Res. 2018 Apr 2:1-5.
Myristica fragrans Houtt., the source of very important spice 'nutmeg' used world over is native to India, Indonesia, Sri Lanka, South Africa and Southeast Asia. Phytochemical investigation of M. fragrans stem bark led to the isolation of bis-aryl dimethyl tetrahydrofuran lignans, such as grandisin [(7S,8S,7'S,8'S)-3,3',4,4',5,5'-hexamethoxy-7,7',8,8'-lignan] and (7S,8S,7'R,8'R)-3,3',4,4',5,5'-hexamethoxy-7,7',8,8'-lignan along with important lignans and neolignans, licarinA, (-)-Licarin B, odoratisol A, (2S, 3R)-7-methoxy-3-methyl-5-((E)-prop-1-enyl)-2-(5-methoxy,3,4-methylenedioxyphenyl) -2,3-dihydrobenzofuran, elemicin, fragransin B1, raphidecursinol B, erythro-(7S,8R)-Delta(8')-4,7-dihydroxy-3,5,3'-trimethoxy-8-O-4'-neolignan, erythro-(7S,8R)-Delta(8)'-7-hydroxy-3,4,3',5'-tetramethoxy-8-O-4'-neolignan, surinamensin.and beta-sitosterol. Structures of the 12 compounds isolated were unambiguously identified by various spectroscopic methods. The former two compounds were isolated from M. fragrans for the first time. Furthermore, the X-ray crystal structure of odoratisol A is reported in this paper for the first time.
HPLC-Guided Isolation, Purification and Characterization of Phenylpropanoid and Phenolic Constituents of Nutmeg Kernel (Myristica fragrans).[Pubmed:27396199]
Nat Prod Commun. 2016 Apr;11(4):483-8.
Many studies on the biological activities of nutmeg continue to appear in the literature. The most common targets include GIT, CNS, oxidative stress and inflammation. However, results obtained from most studies are often inconsistent due to the variability of utilized samples, lack of standardized nutmeg products or insufficient amounts of pure compounds for comprehensive follow-up investigation. To address the consistency and supply issue we utilized available technology to develop a reproducible procedure for preparation of specific extracts and isolation of the major phenolic constituents present in nutmeg kemel. A well-defined and reproducible sequence of extraction, fractionation and chromatographic purification was adopted and was guided by HPLC fingerprinting. Spectroscopic methods, mainly NMR, and mass spectrometry were utilized to identify each compound. Thirteen compounds were isolated in a pure form and identified as: elemicin (1), isoelemicin (2), myristicin (4), surinamensin (5), malabaricone C (6), 2-(3'-allyl-2',6'-dimethoxy-phenyloxy)-l- acetoxy-(3,4-dimethoxyphenyl)-propyl ester (7), methoxylicarin A (8), licarin A (9), malabaricone B (10), licarin C (11), 5'-methoxylicarin B (12), (-)-Licarin B (13), and 2-(3'-allyl-2',6'-dimethoxy-phenyloxy)-l-methyl-5-methoxy-1,2-dihydrobenzofuran (3, a new compound). With repeated isolation runs, these pure compounds can be prepared in quantities sufficient for biological evaluation as needed. The availability of purified compounds will also allow the development of specific, accurate, and sensitive analytical procedures for pharmacokinetic studies and for quality control of nutmeg products. Both aspects are essential for nutmeg-focused drug discovery. The same approach can also be adapted to other medicinal plants of potential interest.
Neolignans from Aristolochia elegans as antagonists of the neurotropic effect of scorpion venom.[Pubmed:25278184]
J Ethnopharmacol. 2014 Nov 18;157:156-60.
ETHNOPHARMACOLOGICAL RELEVANCE: The high frequency of poisoning by sting or bite from venomous animals has begun to be a serious public health problem in Mexico where scorpion sting is the most common. Because of this, there is the need to seek active substances in plant species with an antagonistic effect against neurotropic activity of scorpion venom. The aim of this work was to demonstrate which of the compounds contained in the n-hexane extract from Aristolochia elegans roots display activity against scorpion venom. MATERIAL AND METHODS: Antagonist activity displayed by extract, fractions and isolated compounds obtained from Aristolochia elegans was guided by the inhibition of smooth muscle contraction induced by scorpion venom (Centruroides limpidus limpidus) in a model of isolated guinea pig ileum. The neolignans obtained from this extract were isolated and analyzed by chromatographic methods including HPLC. The chemical characterization of these compounds was performed by the analysis of (1)H and (13)C NMR spectra. RESULTS: The bio-guided chromatographic fractionation allowed us to isolate 4 known neolignans: Eupomatenoid-7 (1), licarin A (2), (-)-Licarin B (3), eupomatenoid-1 (4) and other new neolignan which was characterized as 2-(3'-hydroxy-4'-methoxyphenyl)-3-methyl-5-[(E)-alpha-propen-gamma-al]-7-methoxy- benzo [b] furan (5). This compound was named as eleganal. Compounds 1 and 2 were purified from the most active fraction AeF3 (EC50 of 149.9mug/mL, Emax of 65.66%). A doses-response analysis of eupomatenoid-7(1) and licarin A(2) allowed us to establish EC50 values (65.96mug/mL and 51.96mug/mL) respectively. CONCLUSIONS: The antagonistic effect against Centuroides limpidus limpidus scorpion venom displayed by the n-hexane extract from Aristolochia elegans roots is due to the presence of neolignans 1-2 contained in the fraction AeF3. Chemical analysis of fraction AeF2 allowed the isolation of a new compound which was identified as 2-(3'-hydroxy-4'-methoxyphenyl)-3-methyl-5-[(E)-alpha-propen-gamma-al]-7-methoxy- benzo[b]furan (5), denominated as eleganal.
New inhibitors of nitric oxide production from the seeds of Myristica fragrans.[Pubmed:23994084]
Food Chem Toxicol. 2013 Dec;62:167-71.
Six dihydrobenzofuran type neolignans were isolated from the dried ripe seeds of Myristica fragrans Houtt. (family: Myristicaceae) and their chemical structures were identified as (-)-Licarin B (1), 3'-methoxylicarin B (2), myrisfrageal A (3), isodihydrocainatidin (4), dehydrodiisoeugenol (5), and myrisfrageal B (6), respectively, on the basis of spectroscopic data analyses. Among them, compounds 3 and 6 are new compounds. Compounds 1-6 showed inhibition of nitric oxide production in lipopolysaccharide-activated murine monocyte-macrophage RAW264.7 with IC50 values of 53.6, 48.7, 76.0, 36.0, 33.6, and 45.0 muM, respectively. These values were compared to those of the positive controls, indomethacin and L-N(6)-(1-iminoethyl)-lysine, which have IC50 values of 65.3 and 27.1 muM, respectively. Further compounds 3, 5 and 6 suppressed LPS-induced iNOS mRNA expression in a does-dependent manner in RAW 264.7 cells assayed by real-time RT-PCR. Compounds 3, 5 and 6 may inhibit NO overproduction via inhibition of iNOS mRNA expression. The results provided valuable information for further investigation of compounds 1-6 as anti-inflammatory and chemopreventive agents.
Antimycobacterial neolignans isolated from Aristolochia taliscana.[Pubmed:20209328]
Mem Inst Oswaldo Cruz. 2010 Feb;105(1):45-51.
Tuberculosis (TB - Mycobacterium tuberculosis) is an ancient infectious disease that has appeared once again as a serious worldwide health problem and now comprises the second leading cause of death resulting from a single infection. The prevalence of multidrug resistance (MDR) TB is increasing and therapeutic options for treatment are not always accessible; in fact, some patients do not respond to the available drugs. Therefore, there is an urgent need to develop novel anti-TB agents. The aim of the present study was to screen extracts of Aristolochia taliscana, a plant used in traditional Mexican medicine to treat cough and snake bites, for antimycobacterial activity. The hexanic extract of A. taliscana was tested by microdilution alamar blue assay against Mycobacterium strains and bioguided fractionation led to the isolation of the neolignans licarin A, (-)-Licarin B and eupomatenoid-7, all of which had antimycobacterial activity. Licarin A was the most active compound, with minimum inhibitory concentrations of 3.12-12.5 microg/mL against the following M. tuberculosis strains: H37Rv, four mono-resistant H37Rv variants and 12 clinical MDR isolates, as well as against five non-tuberculous mycobacteria (NTM) strains. In conclusion, licarin A represents a potentially active anti-TB agent to treat MDR M. tuberculosis and NTM strains.
Simultaneous determination of eleven bioactive compounds in Saururus chinensis from different harvesting seasons by HPLC-DAD.[Pubmed:20004543]
J Pharm Biomed Anal. 2010 Apr 6;51(5):1142-6.
A high performance liquid chromatography method coupled with diode array detection (HPLC-DAD) was developed for simultaneous determination of five major active flavonoids, two aristolactams and four main lignans in Saururus chinensis, namely rutin (1), isoquercitrin (2), quercetin-3-O-beta-d-glucopyranosyl (1-->4)-alpha-l-rhamnoside (3), quercitrin (4), quercetin (5), aristolactam A II (6), sauristolactam (7), dihydroguaiaretic acid (8), sauchinone (9), licarin A (10) and (-)-Licarin B (11). The analysis was performed on an Agilent Eclipse XDB C(18) column (4.6mmx150mm, 5microm) with gradient elution of 0.4% aqueous phosphoric acid and acetonitrile. The detection wavelengths were 280 and 360nm. All calibration curves showed good linearity (r(2)>0.9991) within test ranges. The method was reproducible with intra- and inter-day variation less than 3.2%. The recovery of the assay was in the range of 95.1-103.9%. The validated method was successfully applied for the analysis of the eleven bioactive compounds in seven samples from different harvesting seasons and significant variations were revealed. The results indicated that the developed method can be used as a suitable quality control method for S. chinensis and it should be harvested in August (fruiting period) for Jiangsu cultivation regions, taking the yield into consideration, with the highest amounts of lignans, relative higher amounts of flavonoids and lower amounts of aristolactams.
Machilin A isolated from Myristica fragrans stimulates osteoblast differentiation.[Pubmed:19096999]
Planta Med. 2009 Feb;75(2):152-7.
This study evaluated the stimulatory effects of machilin A and structurally related lignans isolated from Myristica fragrans on osteoblast differentiation. In two IN VITRO osteoblast differentiation models, machilin A stimulated osteoblast differentiation via activation of p38 MAP kinase. Lignans isolated from Myristica fragrans also stimulated osteoblast differentiation in MC3T3-E1 cells; the lignans included macelignan, machilin F, nectandrin B, safrole, licarin A, (-)-Licarin B, myristargenol, and meso-dihydroguaiaretic acid. These data suggest that lignans isolated from Myristica fragrans have anabolic activity in bone metabolism.
Increase of caspase-3 activity by lignans from Machilus thunbergii in HL-60 cells.[Pubmed:15305043]
Biol Pharm Bull. 2004 Aug;27(8):1305-7.
Nine lignans and two butanolides were isolated from the stem bark of Machilus thunbergii and their structures were identified as machilin A (1), (-)-Licarin B (2), zuonin B (3), macelignan (4), secoisolancifolide (5), isolancifolide (6), oleiferin C (7), meso-dihydroguaiaretic acid (8), licarin A (9), machilin F (10), and nectandrin B (11) by spectroscopic means. These compounds were assessed for their abilities to activate a caspase-3 activity in human promyeloid leukemic HL-60 cells. The intracellular caspase-3 activity of macelignan (4), oleiferin C (7), meso-dihydroguaiaretic acid (8), and licarin A (9) increased approximately 3.04, 6.16, 2.10, and 3.10-fold at 100 microM over that of untreated control. In addition, compounds 4, 7, 8, and 9 induced internucleosomal DNA fragmentation in HL-60 cells.
Trypanocidal constituents in plants 1. Evaluation of some Mexican plants for their trypanocidal activity and active constituents in Guaco, roots of Aristolochia taliscana.[Pubmed:12230115]
Biol Pharm Bull. 2002 Sep;25(9):1188-91.
Crude extracts of Mexican medicinal plants were screened for trypanocidal activity against Trypanosoma cruzi, which is the etiological agent for Chagas' disease, one of the most serious protozoan diseases in Latin America. There were 43 kinds of methanolic and other organic extracts from 39 plants which were examined by the preliminary screening test to see immobilization of epimastigotes of T. cruzi in vitro. Eighteen of them showed activity at the concentration of 2 mg/ml after incubation for 2 h, while 13 showed activity at the concentration of 1 mg/ml after incubation for 48 h. Among them, the MeOH extract of roots of Aristolochia taliscana (Aristolochiaceae), locally known as "Guaco," immobilized all the epimastigotes even at lower concentration of 0.5 mg/ml (48 h). In order to identify principal compounds for this activity, the MeOH extract of Guaco was subjected to bioassay-guided fractionation. From the active fractions, four neolignans, eupomatenoid-7 (1), licarin A (2), eupomatenoid-1 (5) and (-)-Licarin B (6), and two lignans, austrobailignan-7 (3) and fragransin E1 (4) were isolated. Compounds 1-4 immobilized all the epimastigotes at the minimum concentration of 25-75 microg/ml after incubation for 48 h, while compounds 5 and 6 were inactive. Corresponding concentration of gossypol, berberine chloride and harmine was 280 microg/ml, 300 microg/ml and >500 microg/ml, respectively.
Cytotoxic neolignans and butanolides from Machilus obovatifolia.[Pubmed:11509982]
Planta Med. 2001 Aug;67(6):559-61.
From the chloroform-soluble portion of the stem wood of Machilus obovatifolia, one new neolignan, perseal F (1), four known neolignans, perseal G (2), licarin A, (-)-Licarin B, acuminatin, two butanolides, linderanolide E and isolinderanolide E, two steroids, beta-sitosterol, beta-sitosterol-beta-D-glucoside, and syringaldehyde were isolated. Perseal F (1) and G (2) are neolignans that have a C-1' formyl side chain instead of a propenyl group. Compound 2 was isolated in a mixture with acuminatin. The structure of 2 was identified by comparison with the product formed by the Lemieux-von Rudloff oxidation of (-)-Licarin B. Two minor oxidative by-products, 2a and 2b, were also obtained. Linderanolide E showed cytotoxicities against P-388, KB16, A549 and HT-29, 1 against P-388, KB16 and HT-29, and isolinderanolide E against P-388, cancer cell lines, respectively. All structures were identified by means of spectroscopic analyses.


