FlurbiprofenCyclooxygenase inhibitor CAS# 5104-49-4 |
- Etifoxine
Catalog No.:BCC1560
CAS No.:21715-46-8
- Etomidate
Catalog No.:BCC1150
CAS No.:33125-97-2
- Etifoxine hydrochloride
Catalog No.:BCC1561
CAS No.:56776-32-0
- Acamprosate calcium
Catalog No.:BCC1327
CAS No.:77337-73-6
Quality Control & MSDS
Number of papers citing our products

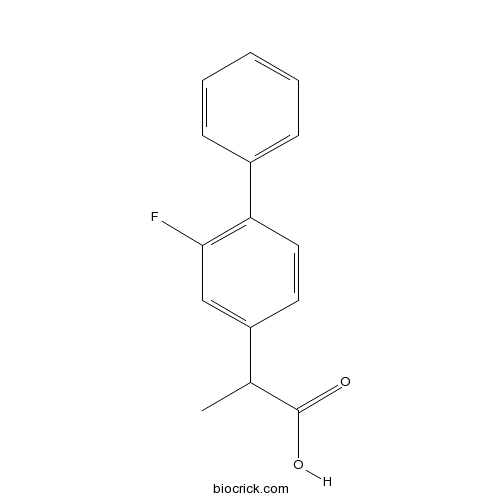
Chemical structure

3D structure
| Cas No. | 5104-49-4 | SDF | Download SDF |
| PubChem ID | 3394 | Appearance | Powder |
| Formula | C15H13FO2 | M.Wt | 244.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | dl-Flurbiprofen | ||
| Solubility | DMSO : ≥ 100 mg/mL (409.40 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-(3-fluoro-4-phenylphenyl)propanoic acid | ||
| SMILES | CC(C1=CC(=C(C=C1)C2=CC=CC=C2)F)C(=O)O | ||
| Standard InChIKey | SYTBZMRGLBWNTM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H13FO2/c1-10(15(17)18)12-7-8-13(14(16)9-12)11-5-3-2-4-6-11/h2-10H,1H3,(H,17,18) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of cyclooxygenase (IC50 values are 0.1 and 0.4 μM for inhibition of human COX-1 and COX-2 respectively). Analgesic, anti-inflammatory and antipyretic in vivo. Inhibits tumor cell growth in vitro and in vivo. Regulates prostate stem cell antigen through activation of Akt kinase. Also inhibits fibroblast proliferation in vitro. |

Flurbiprofen Dilution Calculator

Flurbiprofen Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.094 mL | 20.47 mL | 40.94 mL | 81.88 mL | 102.35 mL |
| 5 mM | 0.8188 mL | 4.094 mL | 8.188 mL | 16.376 mL | 20.47 mL |
| 10 mM | 0.4094 mL | 2.047 mL | 4.094 mL | 8.188 mL | 10.235 mL |
| 50 mM | 0.0819 mL | 0.4094 mL | 0.8188 mL | 1.6376 mL | 2.047 mL |
| 100 mM | 0.0409 mL | 0.2047 mL | 0.4094 mL | 0.8188 mL | 1.0235 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Flurbiprofen is a nonsteroidal anti-inflammatory drug (NSAID) used to relieve symptoms of arthritis (osteoarthritis and rheumatoid arthritis)
- Acipimox
Catalog No.:BCC4884
CAS No.:51037-30-0
- Salbutamol Sulfate
Catalog No.:BCC4338
CAS No.:51022-70-9
- (-)-Licarin B
Catalog No.:BCN1241
CAS No.:51020-87-2
- (+)-Licarin A
Catalog No.:BCC9008
CAS No.:51020-86-1
- Demethylcephalotaxinone
Catalog No.:BCN7070
CAS No.:51020-45-2
- Isocorynoxeine
Catalog No.:BCN5003
CAS No.:51014-29-0
- 6''-O-Malonylgenistin
Catalog No.:BCN2772
CAS No.:51011-05-3
- Minecoside
Catalog No.:BCN5627
CAS No.:51005-44-8
- Galanthaminone
Catalog No.:BCN2867
CAS No.:510-77-0
- AMI-193
Catalog No.:BCC6679
CAS No.:510-74-7
- Echinocystic acid
Catalog No.:BCN5628
CAS No.:510-30-5
- Belladonnine
Catalog No.:BCN1892
CAS No.:510-25-8
- Z-GABA-OH,Z-gama-Abu-OH
Catalog No.:BCC2644
CAS No.:5105-78-2
- alpha-Lipomycin
Catalog No.:BCN1842
CAS No.:51053-40-8
- Wogonoside
Catalog No.:BCN1200
CAS No.:51059-44-0
- ATC 0065
Catalog No.:BCC7666
CAS No.:510732-84-0
- ATC 0175 hydrochloride
Catalog No.:BCC7657
CAS No.:510733-97-8
- Boc-Lys(Z)-pNA
Catalog No.:BCC3419
CAS No.:51078-31-0
- Pyrrolidinedithiocarbamate ammonium
Catalog No.:BCC6766
CAS No.:5108-96-3
- Methyl p-hydroxyphenyllactate
Catalog No.:BCN6669
CAS No.:51095-47-7
- 2,7-Dihydrohomoerysotrine
Catalog No.:BCN5629
CAS No.:51095-85-3
- Nicotine 1'-N-oxide
Catalog No.:BCN6892
CAS No.:51095-86-4
- alpha-Onocerol
Catalog No.:BCN5630
CAS No.:511-01-3
- Sugiol
Catalog No.:BCN3164
CAS No.:511-05-7
Extended release of flurbiprofen from tromethamine-buffered HPMC hydrophilic matrix tablets.[Pubmed:28298171]
Pharm Dev Technol. 2018 Nov;23(9):874-881.
The pH-dependent solubility of a drug can lead to pH-dependent drug release from hydrophilic matrix tablets. Adding buffer salts to the formulation to attempt to mitigate this can impair matrix hydration and negatively impact drug release. An evaluation of the buffering of hydrophilic matrix tablets containing a pH-dependent solubility weak acid drug (Flurbiprofen), identified as possessing a deleterious effect on hydroxypropyl methylcellulose (HPMC) solubility, swelling and gelation, with respect to drug dissolution and the characteristics of the hydrophilic matrix gel layer in the presence of tromethamine as a buffer was undertaken. The inclusion of tromethamine as an alkalizing agent afforded pH-independent Flurbiprofen release from matrices based on both HPMC 2910 (E series) and 2208 (K series), while concomitantly decreasing the apparent critical effect on dissolution mediated by this drug with respect to the early pseudo-gel layer formation and functionality. Drug release profiles were unaffected by matrix pH-changes resulting from loss of tromethamine over time, suggesting that HPMC inhibited precipitation of drug from supersaturated solution in the hydrated matrix. We propose that facilitation of diffusion-based release of potentially deleterious drugs in hydrophilic matrices may be achieved through judicious selection of a buffering species.
Ultrafast Fluorescence Dynamics in Flurbiprofen-Amino Acid Dyads and in the Supramolecular Drug/Protein Complex.[Pubmed:28259191]
Chimia (Aarau). 2017 Feb 22;71(1-2):18-25.
The interaction dynamics between the drug Flurbiprofen (FBP) and human serum albumin (HSA) has been investigated by time-resolved fluorescence spectroscopy, combining femtosecond fluorescence upconversion and picosecond time-correlated single photon counting. In order to obtain additional information on the drug/ protein interaction, several covalently linked model dyads, composed of FBP and tryptophan or tyrosine, were also studied. For all systems, the main feature was a remarkable dynamic FBP fluorescence quenching, more prominent in the dyads than in the protein complex. All systems also displayed a clear stereoselectivity depending on the (S)- or (R)-form of FBP, that was strongly influenced by the conformational arrangement of the investigated chromophores.
Preoperative butorphanol and flurbiprofen axetil therapy attenuates remifentanil-induced hyperalgesia after laparoscopic gynaecological surgery: a randomized double-blind controlled trial.[Pubmed:28077539]
Br J Anaesth. 2016 Oct;117(4):504-511.
BACKGROUND: Several studies indicate that remifentanil exposure may engender opioid-induced hyperalgesia. Butorphanol and Flurbiprofen axetil are proposed as adjunctive analgesics for postoperative pain control. This randomized double-blind controlled study was designed to investigate the antihyperalgesic effects of butorphanol combined with Flurbiprofen axetil on opioid-induced hyperalgesia. METHODS: One hundred and twenty patients undergoing elective laparoscopic gynaecological surgery with sevoflurane anaesthesia were randomized to one of four groups, as follows: intraoperative sufentanil 0.30 microg kg(-1) (Group S); remifentanil 0.30 microg kg(-1) min(-1) (Group R); intraoperative remifentanil and pre-anaesthesia butorphanol 20 microg kg(-1) (Group B); or intraoperative remifentanil and pre-anaesthesia butorphanol 10 microg kg(-1) combined with Flurbiprofen axetil 0.5 mg kg(-1) (Group BF). Sufentanil was used to control postoperative pain. The threshold and area of postoperative mechanical hyperalgesia were measured with Von Frey filaments. Pain intensity, sufentanil consumption, and side-effects were recorded for 24 h after surgery. RESULTS: Compared with Group S, remifentanil anaesthesia increased the pain score, postoperative sufentanil consumption, and area of hyperalgesia [mean 49.9 (sd 8.6) vs 60.5 (10.0) cm(2), P<0.001] and reduced the hyperalgesia threshold on the dominant inner forearm [mean 89.5 (sd 23.4) vs 60.6 (22.6) g, P=0.004]. Compared with Group R, the pain score, sufentanil consumption, and area of hyperalgesia were reduced and hyperalgesia threshold was elevated likewise in Groups B and BF. However, the efficacy in Group BF was higher than in Group B (P=0.021). CONCLUSIONS: The preoperative combination of butorphanol and Flurbiprofen axetil effectively ameliorated opioid-induced hyperalgesia in patients undergoing laparoscopic gynaecological surgery under sevoflurane-remifentanil anaesthesia. CLINICAL TRIAL REGISTRATION: NCT02043366.
Enantioselective Effect of Flurbiprofen on Lithium Disposition in Rats.[Pubmed:28147361]
Pharmacology. 2017;99(5-6):236-239.
AIMS: Lithium is administered for treating bipolar disorders and is mainly excreted into urine. Nonsteroidal anti-inflammatory drugs inhibit this process. In this study, we examined the enantioselective effect of Flurbiprofen on the disposition of lithium in rats. METHODS: Pharmacokinetic experiments with lithium were performed. RESULTS: Until 60 min after the intravenous administration of lithium chloride at 30 mg/kg as a bolus, 17.8% of lithium injected was recovered into the urine. Its renal clearance was calculated to be 1.62 mL/min/kg. Neither creatinine clearance (Ccr) nor pharmacokinetics of lithium was affected by the simultaneous injection of (R)-Flurbiprofen at 20 mg/kg. (S)-Flurbiprofen impaired the renal function and interfered with the urinary excretion of lithium. The ratio of renal clearance of lithium to Ccr was decreased by the (S)-enantiomer. CONCLUSION: This study clarified that the (S)-Flurbiprofen but not (R)-Flurbiprofen inhibited the renal excretion of lithium in rats.
Gene expression profiling in R-flurbiprofen-treated prostate cancer: R-Flurbiprofen regulates prostate stem cell antigen through activation of AKT kinase.[Pubmed:16949054]
Biochem Pharmacol. 2006 Nov 15;72(10):1257-67.
We have used gene expression profiling to characterize genes regulated by the anti-tumor non-steroidal anti-inflammatory drug (NSAID)-like agent R-Flurbiprofen (RFB) in murine TRAMP prostate cancer. Mice with spontaneous, palpable tumors were treated with RFB 25 mg/(kgd) x 7d orally, or vehicle only. RNA was then extracted from tumor tissue and used for microarray analysis with Affymetrix chips. Fifty-eight genes were reproducibly regulated by RFB treatment. One of the most highly up-regulated genes was prostate stem cell antigen (psca). We used TRAMP C1 murine prostate cancer cells to examine potential mechanisms through which RFB could regulate psca. RFB induced dose-dependent expression of PSCA protein, and activity of the psca promoter, in TRAMP C1 cells in culture. Increased psca promoter activity was also seen following treatment of cells with sulindac sulfone, another NSAID-like agent, but not with celecoxib treatment. RFB activation of the psca promoter could be attenuated by co-transfection of dominant-negative akt and h-ras constructs, but not by dominant-negative mek1 plasmids. Immunoblotting revealed that RFB increased expression of phosphorylated AKT at concentrations that stimulated psca promoter activity, and that increased PSCA protein expression. In addition, RFB-dependent up-regulation of PSCA protein expression could be blocked by AKT inhibitors. These data demonstrate that RFB, and possibly other NSAID-like analogs, can increase expression of the psca gene both in vivo and in culture. They further suggest the utility of combining RFB with AKT inhibitors or with monoclonal antibodies targeting PSCA protein, for treatment or prevention of prostate cancer.
The effect of nonsteroidal antiinflammatory drugs ibuprofen, flurbiprofen, and diclofenac on in vitro and in vivo growth of mouse fibrosarcoma.[Pubmed:12094544]
Cancer Invest. 2002;20(4):490-8.
For suppression of primary G:5:113 fibrosarcoma growth, three structurally different cyclooxygenase (COX) inhibitors (ibuprofen, Flurbiprofen, and diclofenac) were administered intraperitoneally (i.p.) in two regimens starting on day 5 after tumor-cell inoculation. Repeated application of 0.15 mg/mouse/day during 14 consecutive days significantly suppressed the tumor growth and increased the percentage of surviving mice. Similar tendency, however without significant differences, was observed when animals were given 0.5 mg/day for five consecutive days. These results suggest that a time schedule of drug application is important for the therapeutic effect. Suppressive effect of diclofenac and Flurbiprofen on tumor growth was also observed under in vitro conditions. We conclude that suppressive effect of these drugs on tumor growth in vivo comprises both direct effects of COX inhibitors on fibrosarcoma cells and indirect effects that are presumably mediated by extratumoral sources. Our findings encourage the use of COX inhibitors in the therapy of fibrosarcoma.
Expression and selective inhibition of the constitutive and inducible forms of human cyclo-oxygenase.[Pubmed:7832763]
Biochem J. 1995 Jan 15;305 ( Pt 2):479-84.
The enzyme cyclo-oxygenase catalyses the oxygenation of arachidonic acid, leading to the formation of prostaglandins. Recently two forms of cyclo-oxygenase have been described: a constitutive (COX-1) enzyme present in most cells and tissues, and an inducible (COX-2) isoenzyme observed in many cells in response to pro-inflammatory cytokines. Constitutive and inducible forms of human cyclo-oxygenase (hCOX-1 and hCOX-2) were cloned and expressed in insect cells, utilizing a baculovirus expression system. hCOX-1 had a specific activity of 18.8 mumol of O2/mg with a Km of 13.8 microM for arachidonate and Vmax. of 1500 nmol of O2/nmol of enzyme, whereas hCOX-2 had a specific activity of 12.2 mumol of O2/mg with a Km of 8.7 microM for arachidonate and a Vmax. of 1090 nmol of O2/nmol of enzyme. Indomethacin inhibited both hCOX-1 and hCOX-2, whereas NS-398 and Dup-697 selectively inhibited hCOX-2. Both NS-398 and Dup-697 exhibited time-dependent inactivation of hCOX-2, as did indomethacin on both enzymes. The competitive inhibitor of hCOX-1, mefenamic acid, also displayed competitive inhibition of hCOX-2. These results demonstrate the ability to generate selective non-steroidal anti-inflammatory drugs (NSAIDs), which could provide useful improvement therapeutically in the treatment of chronic inflammatory disease.


