4'-MethoxyflavoneCAS# 4143-74-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4143-74-2 | SDF | Download SDF |
| PubChem ID | 77793 | Appearance | Powder |
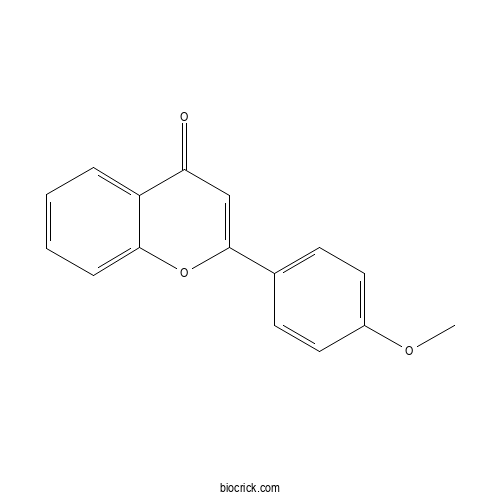
| Formula | C16H12O3 | M.Wt | 252.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(4-methoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC=C(C=C1)C2=CC(=O)C3=CC=CC=C3O2 | ||
| Standard InChIKey | OMICQBVLCVRFGN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O3/c1-18-12-8-6-11(7-9-12)16-10-14(17)13-4-2-3-5-15(13)19-16/h2-10H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4'-Methoxyflavone can inhibit the modulation of the chemiluminescent capacity of macrophages. | |||||

4'-Methoxyflavone Dilution Calculator

4'-Methoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9635 mL | 19.8177 mL | 39.6354 mL | 79.2707 mL | 99.0884 mL |
| 5 mM | 0.7927 mL | 3.9635 mL | 7.9271 mL | 15.8541 mL | 19.8177 mL |
| 10 mM | 0.3964 mL | 1.9818 mL | 3.9635 mL | 7.9271 mL | 9.9088 mL |
| 50 mM | 0.0793 mL | 0.3964 mL | 0.7927 mL | 1.5854 mL | 1.9818 mL |
| 100 mM | 0.0396 mL | 0.1982 mL | 0.3964 mL | 0.7927 mL | 0.9909 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Methyl nonadecanoate
Catalog No.:BCN0033
CAS No.:1731-94-8
- Sutherlandioside B
Catalog No.:BCN0032
CAS No.:1055329-47-9
- 3',4'-Dihydroxyflavone
Catalog No.:BCN0031
CAS No.:4143-64-0
- Saucerneol
Catalog No.:BCN0030
CAS No.:88497-86-3
- (-)-Englerin B
Catalog No.:BCN0029
CAS No.:1094250-13-1
- Kaempferol-7-O-neohesperidoside
Catalog No.:BCN0028
CAS No.:17353-03-6
- (+)-Neomenthol
Catalog No.:BCN0027
CAS No.:2216-52-6
- Terpinolene
Catalog No.:BCN0026
CAS No.:586-62-9
- Nonanal
Catalog No.:BCN0025
CAS No.:124-19-6
- 1'-Acetoxychavicol acetate
Catalog No.:BCN0024
CAS No.:52946-22-2
- 7-Hydroxy-5-methylflavon
Catalog No.:BCN0023
CAS No.:15235-99-1
- 3-Methylindole
Catalog No.:BCN0022
CAS No.:83-34-1
- (1R)-(-)-Menthyl acetate
Catalog No.:BCN0035
CAS No.:2623-23-6
- 8-Acetyl-7-hydroxycoumarin
Catalog No.:BCN0036
CAS No.:6748-68-1
- Steviol 19-glucoside
Catalog No.:BCN0037
CAS No.:64977-89-5
- 1-Octadecanol
Catalog No.:BCN0038
CAS No.:112-92-5
- Myrcene
Catalog No.:BCN0039
CAS No.:123-35-3
- Cucurbitin chloride
Catalog No.:BCN0040
CAS No.:80546-88-9
- Hydroxyvalerenic acid
Catalog No.:BCN0041
CAS No.:1619-16-5
- (+)-D-3-Carene
Catalog No.:BCN0042
CAS No.:498-15-7
- (-)-Hydroxycitric acid lactone
Catalog No.:BCN0043
CAS No.:27750-13-6
- Dhurrin
Catalog No.:BCN0044
CAS No.:499-20-7
- Cimiracemoside F
Catalog No.:BCN0045
CAS No.:264875-61-8
- Englerin A
Catalog No.:BCN0046
CAS No.:1094250-15-3
Whitening Activity of Constituents Isolated from the Trichosanthes Pulp.[Pubmed:32774406]
Evid Based Complement Alternat Med. 2020 Jul 22;2020:2582579.
Whitening cosmetics market has a bright future, and pure natural whitening products of traditional Chinese medicine have always been a research hotspot. In this research, the whitening active ingredient of Chinese medicine Trichosanthes pulp was isolated and purified for the first time, and its whitening mechanism was clarified. Chromatographic methods such as silica gel, ODS, and HPLC were used to isolate and purify them. B16 cells were used to measure the antioxidant activity, tyrosinase activity, and melanin removal activity. A total of 20 compounds were isolated, including p-hydroxybenzaldehyde (1), salicylic acid (2), vanillic acid (3), isovanillic acid (4), protocatechuate (5), trans-cinnamic acid (6), 4-coumaric acid (7), trans-ferulic acid (8), drechslerol-B (9), cyclotucanol 3-palmitate (10), 5-acetoxymethyl-2-furaldehyde (11), 5-hydroxymethylfurfural (12), diosmetin (13), apigenin (14), chrysoeriol (15), luteolin (16), 4'-hydroxyscutellarin (17), quercetin (18), 3',5-dihydroxy-7-(beta-D-glucopyranosyloxy)-4'-methoxyflavone (19), and cofloxacin-7-O-beta-D-glucoside (20). Among them, compounds 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, and 20 have good antioxidant repairing effects; compounds 3, 4, 5, 6, and 7 have high black inhibition; compounds 1, 2, 3, 4, 5, 6, 7, 8, 11, 12, 13, 14, 15, 16, 17, 18, 19, and 20 have obvious tyrosine acidase inhibitory activity. The results laid foundation for the further development and utilization of Trichosanthes pulp resources and also provide a basis for the development of natural whitening cosmetics.
Flavonoids isolated from the South African weed Chromolaena odorata (Asteraceae) have pharmacological activity against uropathogens.[Pubmed:32703212]
BMC Complement Med Ther. 2020 Jul 23;20(1):233.
BACKGROUND: Urinary tract infections (UTIs) caused by opportunistic pathogens are among the leading health challenges globally. Most available treatment options are failing as a result of antibiotic resistance and adverse effects. Natural sources such as plants may serve as promising alternatives. METHODS: Compounds were isolated from the South African weed Chromolaena odorata through column chromatography. Purified compounds were tested for antimicrobial activity using the p-iodonitrotetrazolium chloride (INT) colorimetric method, against uropathogenic Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Aspergillus fumigatus and Cryptococcus neoformans. Anti-biofilm, anti-adhesion and metabolic inhibition activities were investigated against selected strains. Safety of the compounds was determined against Vero monkey kidney, C3A human liver and colon (Caco2) cells. RESULTS: Four compounds identified as pectolinaringenin (1), (+/-)-4',5,7-trimethoxy flavanone (2), 5-hydroxy-3,7,4'-trimethoxyflavone (3) and 3,5,7-trihydroxy-4'-methoxyflavone) (4) were isolated. Minimum inhibitory concentration (MIC) varied between 0.016 and 0.25 mg/mL. Compounds 2 and 3 showed promising antimicrobial activity against E. coli, S. aureus, K. pneumoniae, A. fumigatus and C. neoformans with MIC between 0.016 and 0.125 mg/mL, comparable to gentamicin, ciprofloxacin and amphotericin B used as positive controls. Compounds 2 and 3 showed good anti-biofilm and metabolic inhibition activities against E. coli and S. aureus but weak anti-adhesion activity against the organisms. Low toxicity with selectivity indexes between 1 and 12.625 were recorded with the compounds, indicating that the compounds were rather toxic to the microbial strains and not to the human and animal cells. CONCLUSION: Pharmacological activities displayed by compounds 2 and 3 isolated from C. odorata and low toxicity recorded credits it as a potential lead for the development of useful prophylactic treatments and anti-infective drugs against UTIs. Although known compounds, this is the first time these compounds have been isolated from the South African weed C. odorata and tested for antimicrobial, anti-biofilm, metabolic inhibition and anti-adhesion activities.
Neuroprotective effects of Sophora secundiflora, Sophora tomentosa leaves and formononetin on scopolamine-induced dementia.[Pubmed:32696670]
Nat Prod Res. 2020 Jul 22:1-5.
Five flavonoids were isolated from the ethyl acetate fraction of leaves of Sophora secundiflora; formononetin (1), 5-hydroxy-4'-methoxyflavone (2), genistein (3), 5-hydroxy-8-(1-hydroxy-1-methyl-ethyl)-2-(4-hydroxyphenyl)-4H-furo-[2, 3-h]-chromen-4-one (4) and ononin (5). Additionally, LC-ESI-MS/MS analysis of the ethyl acetate fraction of S. secundiflora leaves had led to tentative identification of eighteen compounds. Formononetin, S. tomentosa and S. secundiflora leaves methanolic extract were evaluated in vivo for their neuroprotective activity where formononetin and S. tomentosa showed promising neuroprotective activity with reduction in acetylcholine esterase (AchE) enzyme activity and elevation of acetylcholine (Ach) and glutathione(GSH) brain levels and attenuation of dopamine (DA), nor-adrenaline (NA) and malonedialdehyde (MDA) brain level significantly, However S. secundiflora leaves methanolic extract didn't attenuate the AchE enzyme activity, DA and NA brain levels. [Formula: see text].
Preference for O-demethylation reactions in the oxidation of 2'-, 3'-, and 4'-methoxyflavones by human cytochrome P450 enzymes.[Pubmed:32312164]
Xenobiotica. 2020 Oct;50(10):1158-1169.
2'-, 3'-, and 4'-Methoxyflavones (MeFs) were incubated with nine forms of recombinant human cytochrome P450 (P450 or CYP) enzymes in the presence of an NADPH-generating system and the products formed were analyzed with LC-MS/MS methods.CYP1B1.1 and 1B1.3 were highly active in demethylating 4'MeF to form 4'-hydroxyflavone (rate of 5.0 nmol/min/nmol P450) and further to 3',4'-dihydroxyflavone (rates of 2.1 and 0.66 nmol/min/nmol P450, respectively). 3'MeF was found to be oxidized by P450s to m/z 239 (M-14) products (presumably 3'-hydroxyflavone) and then to 3',4'-dihydroxyflavone. P450s also catalyzed oxidation of 2'MeF to m/z 239 (M-14) and m/z 255 (M-14, M-14 + 16) products, presumably mono- and di-hydroxylated products, respectively.At least two types of ring oxidation products having m/z 269 fragments were formed, although at slower rates than the formation of mono- and di-hydroxylated products, on incubation of these MeFs with P450s; one type was products oxidized at the C-ring, having m/z 121 fragments, and the other one was the products oxidized at the A-ring (having m/z 137 fragments).Molecular docking analysis indicated the preference of interaction of O-methoxy moiety of methoxyflavones in the active site of CYP1A2.These results suggest that 2'-, 3'-, and 4'-methoxyflavones are principally demethylated by human P450s to form mono- and di-hydroxyflavones and that direct oxidation occurs in these MeFs to form mono-hydroxylated products, oxidized at the A- or B-ring of MeF.
Acacetin enhances glucose uptake through insulin-independent GLUT4 translocation in L6 myotubes.[Pubmed:32126492]
Phytomedicine. 2020 Mar;68:153178.
BACKGROUND: Lowering blood glucose levels by increasing glucose uptake in insulin target tissues, such as skeletal muscle and adipose tissue, is one strategy to discover and develop antidiabetic drugs from natural products used as traditional medicines. PURPOSE: Our goal was to reveal the mechanism and activity of acacetin (5,7-dihydroxy-4'-methoxyflavone), one of the major compounds in Agastache rugose, in stimulating glucose uptake in muscle cells. METHODS: To determine whether acacetin promotes GLUT4-dependent glucose uptake in cultured L6 skeletal muscle cells, we performed a [(14)C] 2-deoxy-D-glucose (2-DG) uptake assay after treating differentiated L6-GLUT4myc cells with acacetin. RESULTS: Acacetin dose-dependently increased 2-DG uptake by enhancing GLUT4 translocation to the plasma membrane. Our results revealed that acacetin activated the CaMKII-AMPK pathway by increasing intracellular calcium concentrations. We also found that aPKClambda/zeta phosphorylation and intracellular reactive oxygen species (ROS) production were involved in acacetin-induced GLUT4 translocation. Moreover, acacetin-activated AMPK inhibited intracellular lipid accumulation and increased 2-DG uptake in HepG2 cells. CONCLUSION: Taken together, these results suggest that acacetin might be useful as an antidiabetic functional ingredient. Subsequent experiments using disease model animals are needed to verify our results.
Inhibition of TGF-beta Signaling in Gliomas by the Flavonoid Diosmetin Isolated from Dracocephalum peregrinum L.[Pubmed:31906574]
Molecules. 2020 Jan 2;25(1). pii: molecules25010192.
Background: Dracocephalum peregrinum L., a traditional Kazakh medicine, has good expectorant, anti-cough, and to some degree, anti-asthmatic effects. Diosmetin (3',5,7-trihydroxy-4'-methoxyflavone), a natural flavonoid found in traditional Chinese herbs, is the main flavonoid in D. peregrinum L. and has been used in various medicinal products because of its anticancer, antimicrobial, antioxidant, estrogenic, and anti-inflammatory effects. The present study aimed to investigate the effects of diosmetin on the proliferation, invasion, and migration of glioma cells, as well as the possible underlying mechanisms. Methods: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), scratch wound, and Transwell assays were used to demonstrate the effects of diosmetin in glioma. Protein levels of Bcl-2, Bax, cleaved caspase-3, transforming growth factor-beta (TGF-beta), E-cadherin, and phosphorylated and unphosphorylated smad2 and smad3 were determined by Western blots. U251 glioma cell development and progression were measured in vivo in a mouse model. Results: Diosmetin inhibited U251 cell proliferation, migration, and invasion in vitro, the TGF-beta signaling pathway, and Bcl-2 expression. In contrast, there was a significant increase in E-cadherin, Bax, and cleaved caspase-3 expression. Furthermore, it effectively reduced the tumorigenicity of glioma cells and promoted apoptosis in vivo. Conclusion: The results of this study suggest that diosmetin suppresses the growth of glioma cells in vitro and in vivo, possibly by activating E-cadherin expression and inhibiting the TGF-beta signaling pathway.
Two new flavones glycosides with antimicrobial activities from Clerodendrum formicarum Gurke (Lamiaceae).[Pubmed:31148483]
Nat Prod Res. 2019 May 31:1-9.
Clerodendrum formicarum Gurke from the Lamiaceae family is a Cameroonian medicinal plant. The crude methanol, methanol residual and ethyl acetate extracts of leaves have been phytochemically studied using chromatography column to afford four compounds; two new flavones glycoside: clerodendronone 1a (3) and clerodendronone 1b (4) along with two known compounds: 5,7-dihydroxy-4'-methoxyflavone (1) and 5-hydroxy-7,4'-dimethoxyflavone (2). Compound structures have been elucidated on the basis of their spectroscopy data and with literature information. The anti-microbial activities of extracts and three isolated compounds were performed. The antibacterial activity was evaluated against four gram positive, five gram negative and three fungus. Clerodendronone 1b (4) showed good antibacterial activity against bacterial gram negative Shigella flexineri NR518 (MIC = 62.5 mug/ml) and moderate activity against Staphylococcus aureus NR46374 (MIC = 250 mu/ml). The ethyl acetate extract recorded good antibacterial activity against Staphylococcus aureus NR46003 (MIC = 125 microg/ml) and Staphylococcus aureus NR46374 (MIC = 125 mug/ml).
Acacetin protects against cerebral ischemia-reperfusion injury via the NLRP3 signaling pathway.[Pubmed:30632500]
Neural Regen Res. 2019 Apr;14(4):605-612.
Acacetin (5,7-dihydroxy-4'-methoxyflavone), a potential neuroprotective agent, has an inhibitory effect on lipopolysaccharide-induced neuroinflammatory reactions. However, whether acacetin has an effect on inflammatory corpuscle 3 (NLRP3) after cerebral ischemia-reperfusion injury has not been fully determined. This study used an improved suture method to establish a cerebral ischemia-reperfusion injury model in C57BL/6 mice. After ischemia with middle cerebral artery occlusion for 1 hour, reperfusion with intraperitoneal injection of 25 mg/kg of acacetin (acacetin group) or an equal volume of saline (0.1 mL/10 g, middle cerebral artery occlusion group) was used to investigate the effect of acacetin on cerebral ischemia-reperfusion injury. Infarct volume and neurological function scores were determined by 2,3,5-triphenyltetrazolium chloride staining and the Zea-Longa scoring method. Compared with the middle cerebral artery occlusion group, neurological function scores and cerebral infarction volumes were significantly reduced in the acacetin group. To understand the effect of acacetin on microglia-mediated inflammatory response after cerebral ischemia-reperfusion injury, immunohistochemistry for the microglia marker calcium adapter protein ionized calcium-binding adaptor molecule 1 (Iba1) was examined in the hippocampus of ischemic brain tissue. In addition, tumor necrosis factor-alpha, interleukin-1beta, and interleukin-6 expression in ischemic brain tissue of mice was quantified by enzyme-linked immunosorbent assay. Expression of Iba1, tumor necrosis factor-alpha, interleukin-1beta and interleukin-6 was significantly lower in the acacetin group compared with the middle cerebral artery occlusion group. Western blot assay results showed that expression of Toll-like receptor 4, nuclear factor kappa B, NLRP3, procaspase-1, caspase-1, pro-interleukin-1beta, and interleukin-1beta were significantly lower in the acacetin group compared with the middle cerebral artery occlusion group. Our findings indicate that acacetin has a protective effect on cerebral ischemia-reperfusion injury, and its mechanism of action is associated with inhibition of microglia-mediated inflammation and the NLRP3 signaling pathway.
Flavonoid glycosides from leaves and straw of Oryza sativa and their effects of cytotoxicity on a macrophage cell line and allelopathic on weed germination.[Pubmed:29556129]
Saudi Pharm J. 2018 Mar;26(3):375-387.
Five new flavonoids namely, 5-hydroxy-6-isoprenyl-7,4'-dimethoxyflavonol-3-O-beta-d-arabinofuranoside (1), 5,7-dihydroxy-4'-methoxyflavone-7-O-beta-d-arabinopyranosyl-2''-n-decan-1'''-oate (2), 3-butanoyl-5,6,8-trihydroxy-7,4'-dimethoxyflavonol--5-O-beta-d-glucopyranoside (3), 7, 4'-dimethoxy-5-hydroxyflavone-5-O-alpha-d-arabinopyranosyl-(2''-->1''')-O-alpha-d -arabinopyranoside (4), and 5,6-dihydroxy-7, 4'-dimethoxyflavone-5-O-alpha-d-glucopyranoside (5), together with two known compounds, were isolated from the methanol extract of Oryza sativa leaves and straw. Their structures of new compounds were elucidated by 1D and 2D NMR spectral methods, viz: COSY, HMBC and HSQC aided by mass techniques and IR spectroscopy. The cytotoxicity of these compounds (1-7) were assessed by using (RAW 264.7) mouse macrophages cell line, and allelopathic effects of compounds (1-7) on the germination characteristics of barnyardgrass (Echinochloa oryzicola) and pigweed (Chenopodium album L.) were also evaluated. The compounds 1, 6 and 7 showed cytotoxicity and compounds 1-7 exhibited significant inhibitory activity on the seed germination of two weed species.
Lignans, flavonoids and coumarins from Viola philippica and their alpha-glucosidase and HCV protease inhibitory activities.[Pubmed:29334261]
Nat Prod Res. 2019 Jun;33(11):1550-1555.
Two lignans including a new one, five flavonoids and five coumarins were isolated from the whole plant of Viola philippica (synonymised as Viola yedoensis Makino). The new compound was structurally determined as (7R,8S,8'S) -3,3'-dimethoxy- 4,4',9-trihydroxy- 7,9'-epoxy-8,8'-lignan 9-O-rutinoside by analysis of its NMR, MS and CD spectroscopic data. The known compounds were characterised by comparing their NMR and MS data with those reported. Among the known compounds, 5-hydroxy-4'-methoxyflavone-7-O- rutinoside, 6,7-di-O-beta-D- glucopyranosylesculetin, and 7R,8S-dihydrodehydrodiconiferyl alcohol 4-O-beta-D- glucopyranoside were isolated and identified from this genus for the first time. Of these compounds, 5-hydroxy-4'-methoxyflavone-7-O-rutinoside and (7R,8S,8'S) -3,3'-dimethoxy- 4,4',9-trihydroxy- 7,9'-epoxy-8,8'-lignan 9-O-rutinoside were potently active against alpha-glucosidase, while the two dimeric coumarins, 5, 5'-bi (6, 7-dihydroxycoumarin) and 6,6',7,7'-tetrahydroxy-5,8'-bicoumarin potently inhibited HCV protease.
neo-Clerodane Diterpenoids from Salvia polystachya Stimulate the Expression of Extracellular Matrix Components in Human Dermal Fibroblasts.[Pubmed:29135252]
J Nat Prod. 2017 Nov 22;80(11):3003-3009.
Eleven neo-clerodane diterpenoids (1-11) including the new analogues 1, 2, and 10, and 3',5,6,7-tetrahydroxy-4'-methoxyflavone (12) were isolated from the aerial parts of Salvia polystachya. Polystachyne G (1) and 15-epi-polystachyne G (2) were isolated as an epimeric mixture, containing a 5-hydroxyfuran-2(5H)-one unit in the side chain at C-12 of the neo-clerodane framework. Polystachyne H (10) contains a 1(10),2-diene moiety and a tertiary C-4 hydroxy group. The structures of these compounds were established by analysis of their NMR spectroscopic and MS spectrometric data. The absolute configurations of compounds 3, 4, and 10 were determined through single-crystal X-ray diffraction analysis. The antibacterial, antifungal, and phytotoxic activities of the diterpenoids were determined. In addition, the stimulatory effect of the expression of extracellular matrix components of nine of the isolates (1-8 and 11) was assayed. Compounds 1-4, 8, and 11 increased the expression of the genes codifying for type I, type III, and type V collagens and for elastin.
Acacetin inhibits neuronal cell death induced by 6-hydroxydopamine in cellular Parkinson's disease model.[Pubmed:29089232]
Bioorg Med Chem Lett. 2017 Dec 1;27(23):5207-5212.
Acacetin (5,7-dihydroxy-4'-methoxyflavone), a flavonoid compound isolated from Flos Chrysanthemi Indici, chrysanthemum, safflower, and Calamintha and Linaria species has been shown to have anti-cancer activity, indicating its potential clinical value in cancer treatment. In this study, we sought to study the potentials of acacetin in preventing human dopaminergic neuronal death via inhibition of 6-hydroxydopamine (6-OHDA)-induced neuronal cell death in the SH-SY5Y cells. Our results suggest that acacetin was effective in preventing 6-OHDA-induced neuronal cell death through regulation of mitochondrial-mediated cascade apoptotic cell death. Pretreatment with acacetin significantly inhibited neurotoxicity and neuronal cell death through reactive oxygen species (ROS) production and mitochondrial membrane potential (MMP) dysfunction. Acacetin also markedly acted on key molecules in apoptotic cell death pathways and reduced phosphorylation of c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinases (PI3K)/Akt, and glycogen synthase kinase-3beta (GSK-3beta). These results suggested that acacetin could inhibit 6-OHDA-induced neuronal cell death originating from ROS-mediated cascade apoptosis pathway. Thus, the results of our study suggest that acacetin is a potent therapeutic agent for PD progression.
Effects of diosmetin on nine cytochrome P450 isoforms, UGTs and three drug transporters in vitro.[Pubmed:28867436]
Toxicol Appl Pharmacol. 2017 Nov 1;334:1-7.
Diosmetin (3', 5, 7-trihydroxy-4'-methoxyflavone), a natural flavonoid from traditional Chinese herbs, has been used in various medicinal products because of its anticancer, antimicrobial, antioxidant, estrogenic and anti-inflammatory activity. However, flavonoids could affect the metabolic enzymes and cause drug-drug interactions (DDI), reducing the efficacy of co-administered drugs and potentially resulting in serious adverse reactions. To evaluate its potential to interact with co-administered drugs, the IC50 value of phase I cytochrome P450 enzymes (CYPs), phase II UDP-glucuronyltransferases (UGTs) and hepatic uptake transporters (organic cation transporters (OCTs), organic anion transporter polypeptides (OATPs) and Na(+)-taurocholate cotransporting polypeptides (NTCPs)) were examined in vitro by LC-MS/MS. Diosmetin showed strong inhibition of CYP1A2 in a concentration-dependent manner. The intensity of the inhibitory effect was followed by CYP2C8, CYP2C9, CYP2C19 and CYP2E1. For CYP2A6, CYP2B6, CYP2D6 and CYP3A4, diosmetin was found to have no significant inhibitory effects, and the induction effect on CYPs was not significant. For UGTs, diosmetin had a minimal inhibitory effect. In addition, the inhibitory effects of diosmetin on OATP and OCT1 were weak, and it had little effect on NTCP. This finding indicated that drug-drug interactions induced by diosmetin may occur through co-administration of drugs metabolized by CYP1A2.
Pharmacologic Inhibition of Autophagy Sensitizes Human Acute Leukemia Jurkat T Cells to Acacetin-Induced Apoptosis.[Pubmed:27817185]
J Microbiol Biotechnol. 2017 Jan 28;27(1):197-205.
Exposure of Jurkat T cell clone (J/Neo cells) to acacetin (5,7-dihydroxy-4'-methoxyflavone), which is present in barnyard millet (Echinochloa esculenta (A. Braun)) grains, caused cytotoxicity, enhancement of apoptotic sub-G1 rate, Bak activation, loss of mitochondrial membrane potential (Deltapsi), activation of caspase-9 and caspase-3, degradation of poly(ADP-ribose) polymerase, and FITC-Annexin V-stainable phosphatidylserine exposure on the external surface of the cytoplasmic membrane without accompanying necrosis. These apoptotic responses were abrogated in Jurkat T cell clone (J/Bcl-xL) overexpressing Bcl-xL. Under the same conditions, cellular autophagic responses, including suppression of the Akt-mTOR pathway and p62/SQSTM1 down-regulation, were commonly detected in J/Neo and J/Bcl-xL cells; however, formation of acridine orange-stainable acidic vascular organelles, LC3-I/II conversion, and Beclin-1 phosphorylation (Ser-15) were detected only in J/Neo cells. Correspondingly, concomitant treatment with the autophagy inhibitor (3-methyladenine or LY294002) appeared to enhance acacetin-induced apoptotic responses, such as Bak activation, Deltapsi loss, activation of caspase-9 and caspase-3, and apoptotic sub-G1 accumulation. This indicated that acacetin could induce apoptosis and cytoprotective autophagy in Jurkat T cells simultaneously. Together, these results demonstrate that acacetin induces not only apoptotic cell death via activation of Bak, loss of Deltapsi, and activation of the mitochondrial caspase cascade, but also cytoprotective autophagy resulting from suppression of the Akt-mTOR pathway. Furthermore, pharmacologic inhibition of the autophagy pathway augments the activation of Bak and resultant mitochondrial damage-mediated apoptosis in Jurkat T cells.


