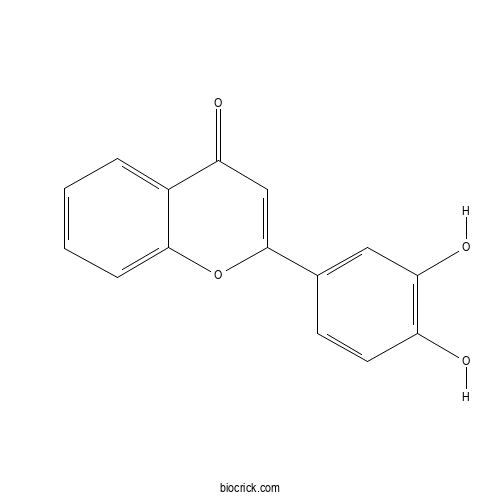
3',4'-DihydroxyflavoneCAS# 4143-64-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4143-64-0 | SDF | Download SDF |
| PubChem ID | 145726 | Appearance | Powder |
| Formula | C15H10O4 | M.Wt | 254.2 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)chromen-4-one | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C=C(O2)C3=CC(=C(C=C3)O)O | ||
| Standard InChIKey | SRNPMQHYWVKBAV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H10O4/c16-11-6-5-9(7-13(11)18)15-8-12(17)10-3-1-2-4-14(10)19-15/h1-8,16,18H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3',4'-Dihydroxyflavone shows anti-inflammatory activity, which might be related to suppression of the proinflammatory MAPK and NF-κB signaling pathways. 3',4'-Dihydroxyflavone can inhibit parasitophorous vacuole formation, modulate glial cell responses, increase NO secretion, and interfere with N. caninum infection and proliferation. | |||||

3',4'-Dihydroxyflavone Dilution Calculator

3',4'-Dihydroxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9339 mL | 19.6696 mL | 39.3391 mL | 78.6782 mL | 98.3478 mL |
| 5 mM | 0.7868 mL | 3.9339 mL | 7.8678 mL | 15.7356 mL | 19.6696 mL |
| 10 mM | 0.3934 mL | 1.967 mL | 3.9339 mL | 7.8678 mL | 9.8348 mL |
| 50 mM | 0.0787 mL | 0.3934 mL | 0.7868 mL | 1.5736 mL | 1.967 mL |
| 100 mM | 0.0393 mL | 0.1967 mL | 0.3934 mL | 0.7868 mL | 0.9835 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Saucerneol
Catalog No.:BCN0030
CAS No.:88497-86-3
- (-)-Englerin B
Catalog No.:BCN0029
CAS No.:1094250-13-1
- Kaempferol-7-O-neohesperidoside
Catalog No.:BCN0028
CAS No.:17353-03-6
- (+)-Neomenthol
Catalog No.:BCN0027
CAS No.:2216-52-6
- Terpinolene
Catalog No.:BCN0026
CAS No.:586-62-9
- Nonanal
Catalog No.:BCN0025
CAS No.:124-19-6
- 1'-Acetoxychavicol acetate
Catalog No.:BCN0024
CAS No.:52946-22-2
- 7-Hydroxy-5-methylflavon
Catalog No.:BCN0023
CAS No.:15235-99-1
- 3-Methylindole
Catalog No.:BCN0022
CAS No.:83-34-1
- Indolelactic acid
Catalog No.:BCN0021
CAS No.:1821-52-9
- Cupressuflavone
Catalog No.:BCN0020
CAS No.:3952-18-9
- Methyl arachidate
Catalog No.:BCN0019
CAS No.:1120-28-1
- Sutherlandioside B
Catalog No.:BCN0032
CAS No.:1055329-47-9
- Methyl nonadecanoate
Catalog No.:BCN0033
CAS No.:1731-94-8
- 4'-Methoxyflavone
Catalog No.:BCN0034
CAS No.:4143-74-2
- (1R)-(-)-Menthyl acetate
Catalog No.:BCN0035
CAS No.:2623-23-6
- 8-Acetyl-7-hydroxycoumarin
Catalog No.:BCN0036
CAS No.:6748-68-1
- Steviol 19-glucoside
Catalog No.:BCN0037
CAS No.:64977-89-5
- 1-Octadecanol
Catalog No.:BCN0038
CAS No.:112-92-5
- Myrcene
Catalog No.:BCN0039
CAS No.:123-35-3
- Cucurbitin chloride
Catalog No.:BCN0040
CAS No.:80546-88-9
- Hydroxyvalerenic acid
Catalog No.:BCN0041
CAS No.:1619-16-5
- (+)-D-3-Carene
Catalog No.:BCN0042
CAS No.:498-15-7
- (-)-Hydroxycitric acid lactone
Catalog No.:BCN0043
CAS No.:27750-13-6
Preference for O-demethylation reactions in the oxidation of 2'-, 3'-, and 4'-methoxyflavones by human cytochrome P450 enzymes.[Pubmed:32312164]
Xenobiotica. 2020 Oct;50(10):1158-1169.
2'-, 3'-, and 4'-Methoxyflavones (MeFs) were incubated with nine forms of recombinant human cytochrome P450 (P450 or CYP) enzymes in the presence of an NADPH-generating system and the products formed were analyzed with LC-MS/MS methods.CYP1B1.1 and 1B1.3 were highly active in demethylating 4'MeF to form 4'-hydroxyflavone (rate of 5.0 nmol/min/nmol P450) and further to 3',4'-dihydroxyflavone (rates of 2.1 and 0.66 nmol/min/nmol P450, respectively). 3'MeF was found to be oxidized by P450s to m/z 239 (M-14) products (presumably 3'-hydroxyflavone) and then to 3',4'-dihydroxyflavone. P450s also catalyzed oxidation of 2'MeF to m/z 239 (M-14) and m/z 255 (M-14, M-14 + 16) products, presumably mono- and di-hydroxylated products, respectively.At least two types of ring oxidation products having m/z 269 fragments were formed, although at slower rates than the formation of mono- and di-hydroxylated products, on incubation of these MeFs with P450s; one type was products oxidized at the C-ring, having m/z 121 fragments, and the other one was the products oxidized at the A-ring (having m/z 137 fragments).Molecular docking analysis indicated the preference of interaction of O-methoxy moiety of methoxyflavones in the active site of CYP1A2.These results suggest that 2'-, 3'-, and 4'-methoxyflavones are principally demethylated by human P450s to form mono- and di-hydroxyflavones and that direct oxidation occurs in these MeFs to form mono-hydroxylated products, oxidized at the A- or B-ring of MeF.
A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3',4'-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study.[Pubmed:31783535]
Molecules. 2019 Nov 27;24(23). pii: molecules24234335.
The beneficial effects of polyphenols, predominantly in the context of oxidative stress-related diseases such as cancer, cardiovascular diseases and neurological conditions including Alzheimer's and Parkinson's diseases, have been documented by a number of papers and reviews. The antioxidant/prooxidant properties of phenolic compounds are related mainly to the number and positions of hydroxyl groups and to their redox metal (Cu, Fe) chelating capacity. In this work we studied structurally distinct phenolic molecules such as myricetin, morin, 3',4'-dihydroxy-flavone, taxifolin and 4-hydroxycoumarin, either alone or as interacting with Cu(2+) ions. EPR and UV-Vis spectroscopy confirmed that the effective binding of cupric ions to phenolic compounds requires the presence of the 3-OH and 4-CO groups on the flavonoid C ring and unsaturated C2-C3 bond of the C-ring, which permits through-conjugation with the B-ring. An ABTS assay revealed that radical scavenging activities of phenolic compounds are related to their number of hydroxyl groups, planarity of the molecular skeleton, extent of delocalization and they decrease in the order: myricetin > morin > 3',4'-dihydroxyflavone ~ 4-hydroxy coumarin > taxifolin. Absorption titrations indicate that copper ions can modulate the DNA binding affinity of flavonoids via the formation of their Cu-chelates. Gel electrophoresis measurements indicated that the protective effect of the phenolic compounds decreases in the order: 3',4'-dihydroxyflavone > 4-OH coumarin > morin > taxifolin ~ myricetin. This can be explained by the fact that myricetin, taxifolin and morin form stable Cu(II) complexes capable of causing DNA damage via interaction with DNA and ROS formation via the Fenton reaction. Application of ROS scavengers revealed the formation of singlet oxygen, superoxide and hydroxyl radicals and their concerted synergistic effect on the DNA. The overall results suggest that the most pronounced DNA damage has been observed for flavonoids containing higher number of hydroxyl groups (including 3-OH group of the C ring), such as myricetin (six hydroxyl groups), morin and taxifolin (five hydroxyl groups) in the presence of Cu(II) ions. The proposed mechanism of action by which Cu(II) complexes of myricetin, morin and taxifolin interact with DNA predispose these substances to act as potential anticancer agents. The anticancer activity of phenolic compounds can be explained by their moderate prooxidant properties, which can boost ROS formation and kill cancer cells. Alternatively, slight prooxidant properties may activate antioxidant systems, including antioxidant enzymes and low molecular antioxidants such as glutathione and thus act as preventive anticancer agents.
Synthetic 3',4'-Dihydroxyflavone Exerts Anti-Neuroinflammatory Effects in BV2 Microglia and a Mouse Model.[Pubmed:29462849]
Biomol Ther (Seoul). 2018 Mar 1;26(2):210-217.
Neuroinflammation is an immune response within the central nervous system against various proinflammatory stimuli. Abnormal activation of this response contributes to neurodegenerative diseases such as Parkinson disease, Alzheimer's disease, and Huntington disease. Therefore, pharmacologic modulation of abnormal neuroinflammation is thought to be a promising approach to amelioration of neurodegenerative diseases. In this study, we evaluated the synthetic flavone derivative 3',4'-dihydroxyflavone, investigating its anti-neuroinflammatory activity in BV2 microglial cells and in a mouse model. In BV2 microglial cells, 3',4'-dihydroxyflavone successfully inhibited production of chemokines such as nitric oxide and prostaglandin E2 and proinflammatory cytokines such as tumor necrosis factor alpha, interleukin 1 beta, and interleukin 6 in BV2 microglia. It also inhibited phosphorylation of mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-kappaB activation. This indicates that the anti-inflammatory activities of 3',4'-dihydroxyflavone might be related to suppression of the proinflammatory MAPK and NF-kappaB signaling pathways. Similar anti-neuroinflammatory activities of the compound were observed in the mouse model. These findings suggest that 3',4'-dihydroxyflavone is a potential drug candidate for the treatment of microglia-related neuroinflammatory diseases.
Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression.[Pubmed:26408079]
Chem Biol Interact. 2015 Dec 5;242:123-38.
The malignant gliomas are very common primary brain tumors with poor prognosis, which require more effective therapies than the current used, such as with chemotherapy drugs. In this work, we investigated the effects of several polyhydroxylated flavonoids namely, rutin, quercetin (F7), apigenin (F32), chrysin (F11), kaempferol (F12), and 3',4'-dihydroxyflavone (F2) in human GL-15 glioblastoma cells. We observed that all flavonoids decreased the number of viable cells and the mitochondrial metabolism. Furthermore, they damaged mitochondria and rough endoplasmic reticulum, inducing apoptosis. Flavonoids also induced a delay in cell migration, related to a reduction in filopodia-like structures on the cell surface, reduction on metalloproteinase (MMP-2) expression and activity, as well as an increase in intra- and extracellular expression of fibronectin, and intracellular expression of laminin. Morphological changes were also evident in adherent cells characterized by the presence of a condensed cell body with thin and long cellular processes, expressing glial fibrillary acidic protein (GFAP). Therefore, these flavonoids should be tested as potential antitumor agents in vitro and in vivo in other malignant glioma models.
Elucidation of hydroxyl groups-antioxidant relationship in mono- and dihydroxyflavones based on O-H bond dissociation enthalpies.[Pubmed:25944672]
J Mol Model. 2015 Jun;21(6):137.
Radical scavenging potential is the key to anti-oxidation of hydroxyflavones which generally found in fruits and vegetables. The objective of this work was to investigate the influence of hydroxyl group on the O-H bond dissociation enthalpies (BDE) from a series of mono- and dihydroxyflavones. Calculation at the B3LYP/6-31G(d,p) level reveals the important roles of an additional one hydroxyl group to boost the BDE of hydroxyflavones that were a stabilization of the generated radicals through attractive H-bond interactions, an ortho- and para-dihydroxyl effect, and a presence of the 3-OH in dihydroxyflavones. On the other hand, the meta-dihydroxyl effect and range-hydroxyl effect especially associated with the either 5-OH or 8-OH promoted greater BDE. Results did not only confirm that dihydroxyflavones had lower BDE than monohydroxyflavones but also suggest the selective potent hydroxyflavone molecules that are the 6'-hydroxyflavone (for monohydroxyflavone) and the 5',6'-, 7,8- and 3',4'-dihydroxyflavone which the corresponding radical preferable generated at C6'-O*, C8-O* and C4'-O*, respectively. Electron distribution was limited only over the two connected rings of hydroxyflavones while the expansion distribution into C-ring could be enhanced if the radical was formed especially for the 2',3'- and 5',6'dihydroxyflavone radicals. The delocalized bonds were strengthened after radical was generated. However the 5-O* in 5,6-dihydroxyflavone and the 3-O* in 3,6'-dihydroxyflavone increased the bond order at C4-O11 which might interrupt the conjugated delocalized bonds at the keto group.
Flavonoids modulate the proliferation of Neospora caninum in glial cell primary cultures.[Pubmed:25548412]
Korean J Parasitol. 2014 Dec;52(6):613-9.
Neospora caninum (Apicomplexa; Sarcocystidae) is a protozoan that causes abortion in cattle, horses, sheep, and dogs as well as neurological and dermatological diseases in dogs. In the central nervous system of dogs infected with N. caninum, cysts were detected that exhibited gliosis and meningitis. Flavonoids are polyphenolic compounds that exhibit antibacterial, antiparasitic, antifungal, and antiviral properties. In this study, we investigated the effects of flavonoids in a well-established in vitro model of N. caninum infection in glial cell cultures. Glial cells were treated individually with 10 different flavonoids, and a subset of cultures was also infected with the NC-1 strain of N. caninum. All of the flavonoids tested induced an increase in the metabolism of glial cells and many of them increased nitrite levels in cultures infected with NC-1 compared to controls and uninfected cultures. Among the flavonoids tested, 3',4'-dihydroxyflavone, 3',4',5,7-tetrahydroxyflavone (luteolin), and 3,3',4',5,6-pentahydroxyflavone (quercetin), also inhibited parasitophorous vacuole formation. Taken together, our findings show that flavonoids modulate glial cell responses, increase NO secretion, and interfere with N. caninum infection and proliferation.
3,3',4',5,5'-Pentahydroxyflavone is a potent inhibitor of amyloid beta fibril formation.[Pubmed:22343025]
Neurosci Lett. 2012 Mar 28;513(1):51-6.
The natural flavonoid fisetin (3,3',4',7-tetrahydroxyflavone) is neurotrophic and prevents fibril formation of amyloid beta protein (Abeta). It is a promising lead compound for the development of therapeutic drugs for Alzheimer's disease. To find even more effective drugs based on the structure of fisetin, we synthesized a series of fisetin analogues lacking the 7-hydroxyl group and compared their effects on Abeta fibril formation determined by the thioflavin T fluorescence assay. 3,3',4'-Trihydroxyflavone and 3',4'-dihydroxyflavone inhibited Abeta fibril formation more potently than fisetin or 3',4',7-trihydroxyflavone, suggesting that the 7-hydroxy group is not necessary for anti-amyloidogenic activity. 3,3',4',5'-Tetrahydroxyflavone and 3',4',5'-trihydroxyflavone inhibited Abeta fibril formation far more potently than 3,3',4'-trihydroxyflavone and 3',4'-dihydroxyflavone, suggesting that 3',4',5'-trihydroxyl group of the B ring is crucial for the anti-amyloidogenic activity of flavonoids. Based on the structure-activity relationship, we synthesized 3,3',4',5,5'-pentahydroxyflavone, and confirmed that this compound is the most potent inhibitor of Abeta fibril formation among fisetin analogues that have been tested. Cytotoxicity assay using rat hippocampal neuron cultures demonstrated that Abeta preincubated with 3,3',4',5,5'-pentahydroxyflavone was significantly less toxic than Abeta preincubated with vehicle. 3,3',4',5,5'-Pentahydroxyflavone could be a new therapeutic drug candidate for the treatment of Alzheimer's disease.
Characterization of an O-methyltransferase from Streptomyces avermitilis MA-4680.[Pubmed:20890103]
J Microbiol Biotechnol. 2010 Sep;20(9):1359-66.
A search of the Streptomyces avermitilis genome reveals that its closest homologs are several O-methyltransferases. Among them, one gene (viz., saomt5) was cloned into the pET-15b expression vector by polymerase chain reaction using sequence-specific oligonucleotide primers. Biochemical characterization with the recombinant protein showed that SaOMT5 was S-adenosyl-L-methionine-dependent Omethyltransferase. Several compounds were tested as substrates of SaOMT5. As a result, SaOMT5 catalyzed Omethylation of flavonoids such as 6,7-dihydroxyflavone, 2',3'-dihydroxyflavone, 3',4'-dihydroxyflavone, quercetin, and 7,8-dihydroxyflavone, and phenolic compounds such as caffeic acid and caffeoyl Co-A. These reaction products were analyzed by TLC, HPLC, LC/MS, and NMR spectroscopy. In addition, SaOMT5 could convert phenolic compounds containing ortho-dihydroxy groups into Omethylated compounds, and 6,7-dihydroxyflavone was known to be the best substrate. SaOMT5 converted 6,7- dihydroxyflavone into 6-hydroxy-7-methoxyflavone and 7-hydroxy-6-methoxyflavone, and caffeic acid into ferulic acid and isoferulic acid, respectively. Moreover, SaOMT5 turned out to be a Mg2+-dependent OMT, and the effect of Mg2+ ion on its activity was five times greater than those of Ca2+, Fe2+, and Cu2+ ions, EDTA, and metal-free medium.
Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization.[Pubmed:19373604]
Nutr Cancer. 2009;61(3):310-21.
Flavonoids are common components of the human diet and appear to be of interest in cancer prevention or therapy, but their structure-activity relationships (SAR) remain poorly defined. In this study, were compared 24 flavonoids for their cytotoxicity on cancer cells (B16 and Lewis lung) and their morphological effect on endothelial cells (EC) that could predict antiangiogenic activity. Ten flavonoids presented inhibitory concentrations for 50% of cancer cells (IC50, 48 h) below 50 microM: rhamnetin, 3',4'-dihydroxyflavone, luteolin, 3-hydroxyflavone, acacetin, apigenin, quercetin, baicalein, fisetin, and galangin. Important SAR for cytotoxicity included the C2-C3 double bond and 3',4'-dihydroxylation. Concerning the morphological effects on EC, only fisetin, quercetin, kaempferol, apigenin, and morin could induce the formation of cell extensions and filopodias at noncytotoxic concentrations. The SAR for morphologic activity differed from cytotoxicity and involved hydroxylation at C-7 and C-4'. Fisetin, the most active agent, presented cell morphology that was distinct compared to colchicine, combretastatin A-4, docetaxel, and cytochalasin D. Resistance to cold depolymerization and a 2.4-fold increase in acetylated alpha-tubulin demonstrated that fisetin was a microtubule stabilizer. In conclusion, this study disclosed several SAR that could guide the choice or the rational synthesis of improved flavonoids for cancer prevention or therapy.
Evaluation of novel chromogenic substrates for the detection of bacterial beta-glucosidase.[Pubmed:17241346]
J Appl Microbiol. 2007 Feb;102(2):410-5.
AIMS: To evaluate three previously unreported substrates for the detection of beta-glucosidase activity in clinically relevant bacteria and to compare their performance with a range of known substrates in an agar medium. METHODS AND RESULTS: The performance of 11 chromogenic beta-glucosidase substrates was compared using 109 Enterobacteriaceae strains, 40 enterococci and 20 strains of Listeria spp. Three previously unreported beta-glucosides were tested including derivatives of alizarin, 3',4'-dihydroxyflavone and 3-hydroxyflavone. These were compared with esculin and beta-glucoside derivatives of 3,4-cyclohexenoesculetin, 8-hydroxyquinoline and five indoxylics. All substrates yielded coloured precipitates upon hydrolysis in agar. Alizarin-beta-D-glucoside was the most sensitive substrate tested and detected beta-glucosidase activity in 72% of Enterobacteriaceae strains and all enterococci and Listeria spp. The two flavone derivatives showed poor sensitivity with Gram-negative bacteria but excellent sensitivity with enterococci and Listeria spp. CONCLUSIONS: Alizarin-beta-d-glucoside is a highly sensitive substrate for detection of bacterial beta-glucosidase and compares favourably with existing substrates. beta-glucosides of 3',4'-dihydroxyflavone and 3-hydroxyflavone are effective substrates for the detection of beta-glucosidase in enterococci and Listeria spp. SIGNIFICANCE AND IMPACT OF THE STUDY: The data presented allow for informed decisions to be made regarding the optimal choice of beta-glucosidase substrate for detection of pathogenic and/or indicator bacteria.
Spectroscopic and theoretical studies of the Zn(II) chelation with hydroxyflavones.[Pubmed:17091955]
J Phys Chem A. 2006 Nov 16;110(45):12494-500.
The Zn(II) complexation of three naturally occurring organic compounds (3-hydroxyflavone, 5-hydroxyflavone, and 3',4'-dihydroxyflavone) has been investigated by electronic spectroscopy combined with quantum chemical calculations. These three ligands, which differ in the nature of their chelating site, lead to the formation of a complex of 1:1 stoichiometry. The experimental results show that it is possible to class the three studied sites, according to their chelating power toward Zn(II), in the following way: alpha-hydroxy-carbonyl > beta-hydroxy-carbonyl > catechol. Time-dependent density functional theory (TD-DFT) calculations were performed to obtain the excitation energies and oscillator strengths of the different complexes. Several effective core potentials (Los Alamos and Stuttgart/Dresden) were used for the description of the Zn ion. Calculations were also performed without any pseudopotential, and they give very satisfying results in the simulation of UV-vis spectra of the three complexes. Only the MWB28 ECP leads globally to a good quality description of the spectral features, roughly comparable to that obtained when the 6-31G(d,p) basis set is used to describe the Zn(II) orbitals. The analysis of the results shows that the nature of electronic transitions involved in the UV-vis spectra greatly differs according to the substitution pattern of the flavonoid.
Time dependent density functional theory study of electronic absorption properties of lead(II) complexes with a series of hydroxyflavones.[Pubmed:16834029]
J Phys Chem A. 2005 Aug 4;109(30):6752-61.
The structural changes occurring with the chelation of lead(II) to 3-hydroxyflavone, 5-hydroxyflavone, and 3',4'-dihydroxyflavone have been investigated by the density functional theory (DFT) method with the B3LYP functional and the 6-31G(d,p) basis set. The two effective core potentials Lanl2dz (Los Alamos) and MWB78 (Stuttgart/Dresden) were used for the Pb ion. Only the 3',4'-dihydroxyflavone ligand shows minor geometrical modifications upon chelation, whereas the two other ligands present important changes of their chromone moiety. The time dependent density functional theory (TD-DFT) has been employed to calculate the electronic absorption spectra of the 1:1 complexes of lead(II) with the three hydroxyflavones, as well in a vacuum as in methanol. The solvent effect is modeled using the self-consistent reaction field (SCRF) method with the polarized continuum model (PCM). Comparison with experimental data allows a precise assessment of the performances of the method, which appears competitive and suitable to reproduce the spectral measurements when the solvent effect is taken into account. These calculations and the molecular orbital analysis have allowed an explanation of the different behaviors of the three ligands toward Pb(II) and particularly the fact that no bathochromic shift is observed with the addition of lead(II) to a 5-hydroxyflavone solution. A complete assignment of the electronic absorption spectra of both free and complexed ligands has been carried out.


