4'-DemethylepipodophyllotoxinAnti-cancer drug CAS# 6559-91-7 |
- Gatifloxacin
Catalog No.:BCC1064
CAS No.:112811-59-3
- Dexrazoxane HCl (ICRF-187, ADR-529)
Catalog No.:BCC1087
CAS No.:149003-01-0
- Doxorubicin (Adriamycin) HCl
Catalog No.:BCC1117
CAS No.:25316-40-9
- Etoposide
Catalog No.:BCC1151
CAS No.:33419-42-0
- Genistein
Catalog No.:BCN5499
CAS No.:446-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6559-91-7 | SDF | Download SDF |
| PubChem ID | 122797 | Appearance | White powder |
| Formula | C21H20O8 | M.Wt | 400.38 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Synonyms | 4'-O-demethylepipodophyllotoxin; 4'-DMEP | ||
| Solubility | DMSO : 100 mg/mL (249.76 mM; Need ultrasonic) | ||
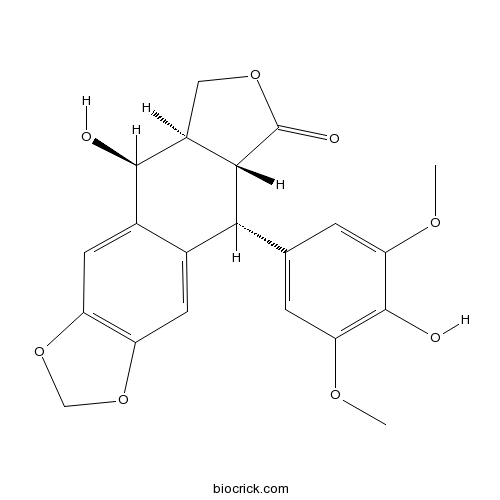
| Chemical Name | (5S,5aR,8aR,9R)-5-hydroxy-9-(4-hydroxy-3,5-dimethoxyphenyl)-5a,6,8a,9-tetrahydro-5H-[2]benzofuro[5,6-f][1,3]benzodioxol-8-one | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C2C3C(COC3=O)C(C4=CC5=C(C=C24)OCO5)O | ||
| Standard InChIKey | YVCVYCSAAZQOJI-JHQYFNNDSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4'-Demethylepipodophyllotoxin is an antimitotic agent which binds to monomeric tubulin, preventing micro-tubule polymerization. It is a potent inhibitor of microtubule assembly. |
| Targets | Topoisomerase |
| In vitro | A rational design strategy of the novel topoisomerase II inhibitors for the synthesis of the 4-O-(2-pyrazinecarboxylic)-4'-demethylepipodophyllotoxin with antitumor activity by diminishing the relaxation reaction of topoisomerase II-DNA decatenation.[Pubmed: 24775914]Bioorg Med Chem. 2014 Jun 1;22(11):2998-3007.A rational design strategy of the novel podophyllum topoisomerase II (Topo II) inhibitors for the synthesis of the esterification and amidation substituted 4'-Demethylepipodophyllotoxin (DMEP) derivates was developed in order to discover the potential antitumor prodrug. Firstly, according to the structure-activity relationship, drug combination principle and bioisosterism, the -COO- and the -NH- bond substituents at the 4 position of cycloparaffin would be a great modification direction to improve antitumor activity of 4'-Demethylepipodophyllotoxin (DMEP). Secondly, from the prodrug principle view, the esterification and amidation at the C-4 position of DMEP would be two useful structure modifications for improve solubility. Thirdly, from the activity pocket in Topo II-DNA cleavage complex point of view, a series of heterocyclic with pharmacological activity were chosen as module for improving antitumor activity by binding with Topo II. Finally, nine novel esterification and amidation DMEP derivates were designed and synthesized for the potential Topo II inhibitors with the superior biological activity.
|
| Kinase Assay | Antitumor agents. 111. New 4-hydroxylated and 4-halogenated anilino derivatives of 4'-demethylepipodophyllotoxin as potent inhibitors of human DNA topoisomerase II.[Pubmed: 2158562]J Med Chem. 1990 May;33(5):1364-8.A series of C-4 hydroxylated and halogenated anilino derivatives of epipodophyllotoxin or 4'-Demethylepipodophyllotoxin have been synthesized and evaluated for their inhibitory activity against the human DNA topoisomerase II as well as for their activity in causing cellular protein-linked DNA breakage. Compounds 11-17 and 22 are more potent than etoposide in causing DNA breakage, while compounds 11-13, 15, 16, and 20 are as active or more active than etoposide in their inhibition of the human DNA topoisomerase II. The cytotoxicity in KB cells appears to have no direct correlation with their ability to inhibit DNA topoisomerase II and to cause protein-linked DNA breaks in cells. |

4'-Demethylepipodophyllotoxin Dilution Calculator

4'-Demethylepipodophyllotoxin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4976 mL | 12.4881 mL | 24.9763 mL | 49.9525 mL | 62.4407 mL |
| 5 mM | 0.4995 mL | 2.4976 mL | 4.9953 mL | 9.9905 mL | 12.4881 mL |
| 10 mM | 0.2498 mL | 1.2488 mL | 2.4976 mL | 4.9953 mL | 6.2441 mL |
| 50 mM | 0.05 mL | 0.2498 mL | 0.4995 mL | 0.9991 mL | 1.2488 mL |
| 100 mM | 0.025 mL | 0.1249 mL | 0.2498 mL | 0.4995 mL | 0.6244 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Description: IC50 Value: 0.31uM(EC50 for HL60 cell, MTT assay, 48h); 0.37uM(EC50 for HepG2 cell, MTT assay, 48h) [1] 4'-demethylepipodophyllotoxin is a key intermediate compounds for the preparation of podophyllotoxin-type anti-cancer drugs. in vitro: 4-TMP-DMEP showed strong cytotoxicity activity against the above-mentioned five tumor cell lines. The EC50s of 4-TMP-DMEP against these tumor cell lines ranged from 0.24 to 0.11 μM, which were 0.29 to 3618 times lower than that of DMEP [1]. in vivo: Treatment of animals with DMEP (until the end of the experiment), 30 min before TPA treatment, significantly reduced the tumor incidence, tumor volume and the conversion efficiency of papillomas to squamous cell carcinomas. The tumor formation and growth was also delayed by DMEP pre-treatment [2]. Clinical trial: N/A
- 1-Hydroxyacridone
Catalog No.:BCN7524
CAS No.:65582-54-9
- Europine N-oxide
Catalog No.:BCN1977
CAS No.:65582-53-8
- Cyclo(Pro-Ala)
Catalog No.:BCN2427
CAS No.:65556-33-4
- Esculentoside A
Catalog No.:BCN5010
CAS No.:65497-07-6
- Naftifine HCl
Catalog No.:BCC4806
CAS No.:65473-14-5
- (Z)-23-Coumaroylhederagenin
Catalog No.:BCN3748
CAS No.:654678-61-2
- Sitagliptin phosphate
Catalog No.:BCC9148
CAS No.:654671-78-0
- Sitagliptin phosphate monohydrate
Catalog No.:BCC2111
CAS No.:654671-77-9
- Fmoc-Asn(Trt)-ol
Catalog No.:BCC2585
CAS No.:654670-89-0
- Acantrifoic acid A
Catalog No.:BCN6488
CAS No.:654663-85-1
- Phebalosin
Catalog No.:BCN4198
CAS No.:6545-99-9
- 1-Methoxyphaseollidin
Catalog No.:BCN7185
CAS No.:65428-13-9
- Cerbinal
Catalog No.:BCN4199
CAS No.:65597-42-4
- Cerberic acid
Catalog No.:BCN4200
CAS No.:65597-44-6
- Ophiopogonin D'
Catalog No.:BCN2645
CAS No.:65604-80-0
- Nintedanib (BIBF 1120)
Catalog No.:BCC3661
CAS No.:656247-17-5
- Fenretinide
Catalog No.:BCC1572
CAS No.:65646-68-6
- Esculentoside E
Catalog No.:BCN5014
CAS No.:65649-36-7
- Silybin B maltoside
Catalog No.:BCC8250
CAS No.:335299-49-5
- HA14-1
Catalog No.:BCC3593
CAS No.:65673-63-4
- AG-1024
Catalog No.:BCC1242
CAS No.:65678-07-1
- TC-C 14G
Catalog No.:BCC6144
CAS No.:656804-72-7
- Reversine
Catalog No.:BCC1892
CAS No.:656820-32-5
- Metformin
Catalog No.:BCC9026
CAS No.:657-24-9
Antitumor agents. 111. New 4-hydroxylated and 4-halogenated anilino derivatives of 4'-demethylepipodophyllotoxin as potent inhibitors of human DNA topoisomerase II.[Pubmed:2158562]
J Med Chem. 1990 May;33(5):1364-8.
A series of C-4 hydroxylated and halogenated anilino derivatives of epipodophyllotoxin or 4'-demethylepipodophyllotoxin have been synthesized and evaluated for their inhibitory activity against the human DNA topoisomerase II as well as for their activity in causing cellular protein-linked DNA breakage. Compounds 11-17 and 22 are more potent than etoposide in causing DNA breakage, while compounds 11-13, 15, 16, and 20 are as active or more active than etoposide in their inhibition of the human DNA topoisomerase II. The cytotoxicity in KB cells appears to have no direct correlation with their ability to inhibit DNA topoisomerase II and to cause protein-linked DNA breaks in cells.
A rational design strategy of the novel topoisomerase II inhibitors for the synthesis of the 4-O-(2-pyrazinecarboxylic)-4'-demethylepipodophyllotoxin with antitumor activity by diminishing the relaxation reaction of topoisomerase II-DNA decatenation.[Pubmed:24775914]
Bioorg Med Chem. 2014 Jun 1;22(11):2998-3007.
A rational design strategy of the novel podophyllum topoisomerase II (Topo II) inhibitors for the synthesis of the esterification and amidation substituted 4'-demethylepipodophyllotoxin (DMEP) derivates was developed in order to discover the potential antitumor prodrug. Firstly, according to the structure-activity relationship, drug combination principle and bioisosterism, the -COO- and the -NH- bond substituents at the 4 position of cycloparaffin would be a great modification direction to improve antitumor activity of 4'-demethylepipodophyllotoxin (DMEP). Secondly, from the prodrug principle view, the esterification and amidation at the C-4 position of DMEP would be two useful structure modifications for improve solubility. Thirdly, from the activity pocket in Topo II-DNA cleavage complex point of view, a series of heterocyclic with pharmacological activity were chosen as module for improving antitumor activity by binding with Topo II. Finally, nine novel esterification and amidation DMEP derivates were designed and synthesized for the potential Topo II inhibitors with the superior biological activity. All the novel compounds exhibited promising in vitro antitumor activity, especially 4-O-(2-pyrazinecarboxylic)-4'-demethylepipodophyllotoxin (compound 1). The antitumor activity of compound 1 against tumor cell line HeLa (i.e., the IC50 value of 0.60 +/- 0.20 muM), A549 (i.e., the IC50 value of 3.83 +/- 0.08 muM), HepG2 (i.e., the IC50 value of 1.21 +/- 0.05 muM), and BGC-823 (i.e., the IC50 value of 4.15 +/- 1.13 muM) was significantly improved by 66, 16, 12, and 6 times than that of the clinically important podophyllum anticancer drug etoposide (i.e., the IC50 values of 15.32 +/- 0.10, 59.38 +/- 0.77, 67.25 +/- 7.05, and 30.74 +/- 5.13 muM), respectively. Compound 1 could arrest HeLa cell cycle G2/M and induce apoptosis by strongly diminishing the relaxation reaction of Topo II-DNA decatenation. The correctness of rational drug design was strictly demonstrated by the bioactivity test.
Synthesis and cytotoxic activity of novel derivatives of 4'-demethylepipodophyllotoxin.[Pubmed:15380199]
Bioorg Med Chem Lett. 2004 Oct 18;14(20):5063-6.
Nine novel 4beta-N-substituted-5-FU-4'-demethylepipodophyllotoxin derivatives were synthesized and evaluated as potential antitumor agents. All of the target compounds showed more significant cytotoxic activity against HL-60 and A-549 in vitro than VP-16 and 5-FU. Among them, 4beta-N-substituted-phenylalanine 5-Fu pentyl ester-4'-demethylepipodophyllotoxin (9 g) was found to exhibit most potent cytotoxic activity against HL-60 and A-549 cell (IC50 is 0.04 and <0.01 microM, respectively).


