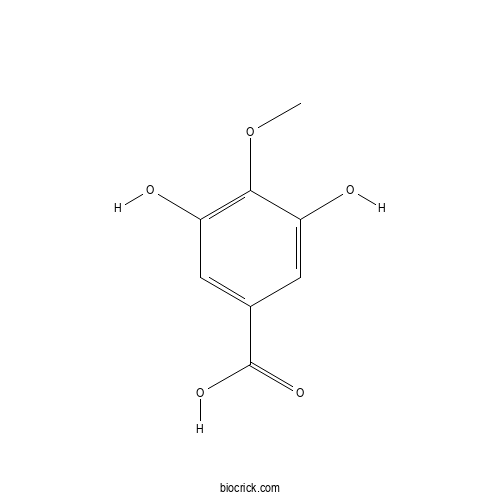
3,5-Dihydroxy-4-methoxybenzoic acidCAS# 4319-02-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4319-02-2 | SDF | Download SDF |
| PubChem ID | 78016 | Appearance | Powder |
| Formula | C8H8O5 | M.Wt | 184.1 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,5-dihydroxy-4-methoxybenzoic acid | ||
| SMILES | COC1=C(C=C(C=C1O)C(=O)O)O | ||
| Standard InChIKey | UBXDWYFLYYJQFR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H8O5/c1-13-7-5(9)2-4(8(11)12)3-6(7)10/h2-3,9-10H,1H3,(H,11,12) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 3,5-dihydroxy-4-methoxybenzoic acid is a COMT inhibitor. | |||||

3,5-Dihydroxy-4-methoxybenzoic acid Dilution Calculator

3,5-Dihydroxy-4-methoxybenzoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4318 mL | 27.1592 mL | 54.3183 mL | 108.6366 mL | 135.7958 mL |
| 5 mM | 1.0864 mL | 5.4318 mL | 10.8637 mL | 21.7273 mL | 27.1592 mL |
| 10 mM | 0.5432 mL | 2.7159 mL | 5.4318 mL | 10.8637 mL | 13.5796 mL |
| 50 mM | 0.1086 mL | 0.5432 mL | 1.0864 mL | 2.1727 mL | 2.7159 mL |
| 100 mM | 0.0543 mL | 0.2716 mL | 0.5432 mL | 1.0864 mL | 1.358 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Nortrachelogenin-5'-C-beta-glucoside
Catalog No.:BCN9775
CAS No.:858127-39-6
- Glucofrangulin A
Catalog No.:BCN9774
CAS No.:21133-53-9
- DL-Tyrosine
Catalog No.:BCN9773
CAS No.:556-03-6
- 4'-Methoxychalcone
Catalog No.:BCN9772
CAS No.:959-23-9
- (-)-Eburnamonine
Catalog No.:BCN9771
CAS No.:4880-88-0
- Comanthosid A
Catalog No.:BCN9770
CAS No.:70938-59-9
- 2'-Methoxyflavone
Catalog No.:BCN9769
CAS No.:19725-47-4
- N-trans-caffeoyloctopamine
Catalog No.:BCN9768
CAS No.:1378868-10-0
- Rebaudioside M
Catalog No.:BCN9767
CAS No.:1220616-44-3
- (S)-(-)-Limonene
Catalog No.:BCN9766
CAS No.:5989-54-8
- L-(-)-Malic acid
Catalog No.:BCN9765
CAS No.:97-67-6
- Glucofrangulin B
Catalog No.:BCN9764
CAS No.:14062-59-0
- 3,4-Dimethoxyacetophenone
Catalog No.:BCN9777
CAS No.:1131-62-0
- Calvatic acid
Catalog No.:BCN9778
CAS No.:54723-08-9
- alpha-Hexylcinnamaldehyde
Catalog No.:BCN9779
CAS No.:101-86-0
- (+/-)-Anabasine
Catalog No.:BCN9780
CAS No.:13078-04-1
- Cannabisin B
Catalog No.:BCN9781
CAS No.:144506-17-2
- Deacylgymnemic acid
Catalog No.:BCN9782
CAS No.:121686-42-8
- Cinobufotenine
Catalog No.:BCN9783
CAS No.:60657-23-0
- Indole
Catalog No.:BCN9784
CAS No.:120-72-9
- 5,6,7-Trimethoxyflavone
Catalog No.:BCN9785
CAS No.:973-67-1
- 3-Methyl-1-butanol
Catalog No.:BCN9786
CAS No.:123-51-3
- Quercetin 3-O-beta-D-glucosyl-(1->2)-rhamnoside
Catalog No.:BCN9787
CAS No.:143016-74-4
- Ethyl phenylacetate
Catalog No.:BCN9788
CAS No.:101-97-3
Anti-Inflammatory Principles from Tamarix aphylla L.: A Bioassay-Guided Fractionation Study.[Pubmed:32630007]
Molecules. 2020 Jun 30;25(13). pii: molecules25132994.
Natural products have served as primary remedies since ancient times due to their cultural acceptance and outstanding biodiversity. To investigate whether Tamarix aphylla L. modulates an inflammatory process, we carried out bioassay-guided isolation where the extracts and isolated compounds were tested for their modulatory effects on several inflammatory indicators, such as nitric oxide (NO), reactive oxygen species (ROS), proinflammatory cytokine; tumour necrosis factor (TNF-alpha), as well as the proliferation of the lymphocyte T-cells. The aqueous ethanolic extract of the plant inhibited the intracellular ROS production, NO generation, and T-cell proliferation. The aqueous ethanolic crude extract was partitioned by liquid-liquid fractionation using n-hexane (n-C6H6), dichloromethane (DCM), ethyl acetate (EtOAc), n-butanol (n-BuOH), and water (H2O). The DCM and n-BuOH extracts showed the highest activity against most inflammatory indicators and were further purified to obtain compounds 1-4. The structures of 3,5-dihydroxy-4',7-dimethoxyflavone (1) and 3,5-Dihydroxy-4-methoxybenzoic acid methyl ester (2) from the DCM extracts; and kaempferol (3), and 3-hydroxy-4-methoxy-(E)-cinnamic acid (4) from the n-BuOH extract were elucidated by different spectroscopic tools, including MS, NMR, UV, and IR. Compound 2 inhibited the production of ROS and TNF-alpha, whereas compound 3 showed inhibitory activity against all the tested mediators. A better understanding of the potential aspect of Tamarix aphylla L. derivatives as anti-inflammatory agents could open the door for the development of advanced anti-inflammatory entities.
Structure-Activity Relationships of Antimicrobial Gallic Acid Derivatives from Pomegranate and Acacia Fruit Extracts against Potato Bacterial Wilt Pathogen.[Pubmed:26080741]
Chem Biodivers. 2015 Jun;12(6):955-62.
Bacterial wilts of potato, tomato, pepper, and or eggplant caused by Ralstonia solanacearum are among the most serious plant diseases worldwide. In this study, the issue of developing bactericidal agents from natural sources against R. solanacearum derived from plant extracts was addressed. Extracts prepared from 25 plant species with antiseptic relevance in Egyptian folk medicine were screened for their antimicrobial properties against the potato pathogen R. solancearum by using the disc-zone inhibition assay and microtitre plate dilution method. Plants exhibiting notable antimicrobial activities against the tested pathogen include extracts from Acacia arabica and Punica granatum. Bioactivity-guided fractionation of A. arabica and P. granatum resulted in the isolation of bioactive compounds 3,5-Dihydroxy-4-methoxybenzoic acid and gallic acid, in addition to epicatechin. All isolates displayed significant antimicrobial activities against R. solanacearum (MIC values 0.5-9 mg/ml), with 3,5-Dihydroxy-4-methoxybenzoic acid being the most effective one with a MIC value of 0.47 mg/ml. We further performed a structure-activity relationship (SAR) study for the inhibition of R. solanacearum growth by ten natural, structurally related benzoic acids.
Chemical constituents from the roots of Leea thorelii Gagnep.[Pubmed:24784484]
Nat Prod Res. 2014;28(13):1015-7.
Phytochemical investigation of the roots of Leea thorelii led to the isolation of nine compounds. Their structures were determined from spectroscopic data as bergenin (1), 11-O-acetyl bergenin (2), 11-O-(4'-O-methylgalloyl) bergenin (3), 3,5-Dihydroxy-4-methoxybenzoic acid (4), ( - )-epicatechin (5), 4"-O-methyl-( - )-epicatechin gallate (6), ( - )-epicatechin gallate (7), microminutinin (8) and stigmasterol. Compounds 1-8 are reported for the first time from this plant, and this is also the first report of the presence of 1, 3, 4, 6 and 8 in the Vitaceae family.
Conjugates of 3alpha-methoxyserrat-14-en-21beta-ol (PJ-1) and 3beta-methoxyserrat-14-en-21beta-ol (PJ-2) as cancer chemopreventive agents.[Pubmed:21620532]
Eur J Med Chem. 2011 Aug;46(8):3368-75.
3alpha-Methoxyserrat-14-en-21beta-ol (PJ-1) and 3beta-methoxyserrat-14-en-21beta-ol (PJ-2) were conjugated with well-known phenolic compounds, narigenin, hesperetin, genistein, and daidzein (1-8). Other conjugates of PJ-2-3,5-Dihydroxy-4-methoxybenzoic acid (9), PJ-2-pyrogallol (10), and derivatives of PJ-1, PJ-2-3,3-dimethyl-succinates (11, 12), PJ-1, PJ-2-succinates (13, 14), PJ-2-glycine (15), PJ-2-piperidine acetic acid (16), and PJ-1 epoxy-3,3-dimethyl-succinate (17) were tested for their inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) activation induced by 12-O-tetradecanoylphorbol-13-acetate (TPA). The inhibitory effects of 11 (IC(50) = 251), 12 (IC(50) = 248), and 17 (IC(50) = 230 mol ratio/32 pmol/TPA), were 2-fold stronger than those of the other compounds such as oleanolic acid (IC(50) = 449). Compounds 10, 11, and 17 inhibited mouse skin tumor promotion in an in vivo two-stage carcinogenesis model. The in vivo two-stage mouse-skin carcinogenesis test employed 7,12-dimethylbenz[a]anthracene (DMBA) as an initiator and TPA as a promoter.


