[Ser25] Protein Kinase C (19-31)PKC substrate CAS# 136795-05-6 |
![[Ser25] Protein Kinase C (19-31) [Ser25] Protein Kinase C (19-31)](/media/images/struct/BCC1022.png)
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 136795-05-6 | SDF | Download SDF |
| PubChem ID | 25085169 | Appearance | Powder |
| Formula | C67H118N26O17 | M.Wt | 1559.82 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Sequence | H2N-Arg-Phe-Ala-Arg-Lys-Gly-Ser-Leu-Arg-Gln-Lys-Asn-Val-OH | ||
| Chemical Name | (2S)-2-[[(2S)-4-amino-2-[[(2S)-6-amino-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-3-phenylpropanoyl]amino]propanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]hexanoyl]amino]acetyl]amino]-3-hydroxypropanoyl]amino]-4-methylpentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]amino]-5-oxopentanoyl]amino]hexanoyl]amino]-4-oxobutanoyl]amino]-3-methylbutanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(CCCN=C(N)N)C(=O)NC(CCC(=O)N)C(=O)NC(CCCCN)C(=O)NC(CC(=O)N)C(=O)NC(C(C)C)C(=O)O)NC(=O)C(CO)NC(=O)CNC(=O)C(CCCCN)NC(=O)C(CCCN=C(N)N)NC(=O)C(C)NC(=O)C(CC1=CC=CC=C1)NC(=O)C(CCCN=C(N)N)N | ||
| Standard InChIKey | JLTBKSGYPRXXGI-WMJPZMSUSA-N | ||
| Standard InChI | InChI=1S/C67H118N26O17/c1-35(2)30-45(61(106)88-43(22-15-29-81-67(77)78)57(102)89-44(23-24-49(71)95)59(104)87-41(20-10-12-26-69)58(103)92-47(32-50(72)96)62(107)93-52(36(3)4)64(109)110)91-63(108)48(34-94)84-51(97)33-82-55(100)40(19-9-11-25-68)86-56(101)42(21-14-28-80-66(75)76)85-53(98)37(5)83-60(105)46(31-38-16-7-6-8-17-38)90-54(99)39(70)18-13-27-79-65(73)74/h6-8,16-17,35-37,39-48,52,94H,9-15,18-34,68-70H2,1-5H3,(H2,71,95)(H2,72,96)(H,82,100)(H,83,105)(H,84,97)(H,85,98)(H,86,101)(H,87,104)(H,88,106)(H,89,102)(H,90,99)(H,91,108)(H,92,103)(H,93,107)(H,109,110)(H4,73,74,79)(H4,75,76,80)(H4,77,78,81)/t37-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,52-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

[Ser25] Protein Kinase C (19-31) Dilution Calculator

[Ser25] Protein Kinase C (19-31) Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Protein Kinase C (19-31), (C67H118N26O17), a peptide with the sequence H-ARG-PHE-ALA-ARG-LYS-GLY-SER-LEU-ARG-GLN-LYS-ASN-VAL-OH, MW= 1559.82. this peptide derived from the pseudo-substrate regulatory domain of PKCa (residues 19-31) with a serine at position 25 replacing the wild-type alanine, it was used as protein kinase C substrate peptide for testing the protein kinase C activity.
Protein kinase C also known as PKC, is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins. PKCa has been reported to play roles in many different cellular processes, such as cell adhesion, cell transformation, cell cycle checkpoint, and cell volume control. Knockout studies in mice suggest that this kinase may be a fundamental regulator of cardiac contractility and Ca2+ handling in myocytes.
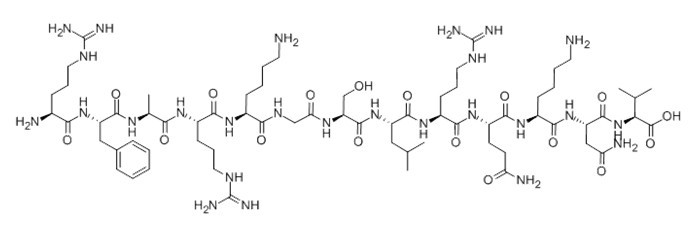
Figure1 Formula of [Ser25] Protein Kinase C (19-31)
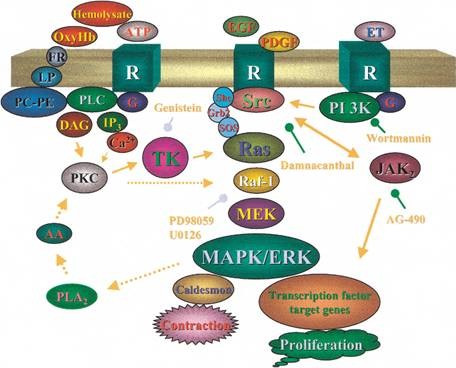
Figure2 Signaling pathway of Protein Kinase C
References:
1. Mellor H, Parker PJ (1998). "The extended protein kinase C superfamily". Biochem. J.. 332 ( Pt 2): 281–92
2. Nishizuka Y (1995). "Protein kinase C and lipid signaling for sustained cellular responses" (abstract). FASEB J. 9 (7): 484–96.
3. Vicente Micol. Correlation between Protein Kinase C an Activity and Membrane Phase Behavior. Departamento de Bioquı´mica y Biologı´a Molecular
- Lubiprostone
Catalog No.:BCC4918
CAS No.:136790-76-6
- Goniodiol diacetate
Catalog No.:BCN3956
CAS No.:136778-40-0
- Retinyl glucoside
Catalog No.:BCC1891
CAS No.:136778-12-6
- Gallic aldehyde
Catalog No.:BCN7859
CAS No.:13677-79-7
- 4,4'-Bismaleimidodiphenylmethane
Catalog No.:BCC8662
CAS No.:13676-54-5
- Minimolide F
Catalog No.:BCN6424
CAS No.:1367351-41-4
- 2-Mercaptoethanesulfonic acid
Catalog No.:BCC1789
CAS No.:3375-50-6
- NB-598 hydrochloride
Catalog No.:BCC1787
CAS No.:136719-25-0
- 9-Deoxygoniopypyrone
Catalog No.:BCN3931
CAS No.:136685-37-5
- PD 123319 ditrifluoroacetate
Catalog No.:BCC1841
CAS No.:136676-91-0
- MK-0591
Catalog No.:BCC1753
CAS No.:136668-42-3
- Timosaponin BII
Catalog No.:BCN4998
CAS No.:136656-07-0
- 6-O-Cinnamoylcatalpol
Catalog No.:BCN6193
CAS No.:136807-41-5
- Delavirdine
Catalog No.:BCC4300
CAS No.:136817-59-9
- 1-Dehydro-10-gingerdione
Catalog No.:BCN3338
CAS No.:136826-50-1
- TOTU
Catalog No.:BCC2826
CAS No.:136849-72-4
- Macranthoidin B
Catalog No.:BCN5938
CAS No.:136849-88-2
- AHU-377 hemicalcium salt
Catalog No.:BCC5141
CAS No.:1369773-39-6
- (-)-Syringaresnol-4-O-beta-D-apiofuranosyl-(1->2)-beta-D-glucopyranoside
Catalog No.:BCN1578
CAS No.:136997-64-3
- Sophoraflavanone H
Catalog No.:BCN6864
CAS No.:136997-68-7
- Sophoraflavanone I
Catalog No.:BCN6863
CAS No.:136997-69-8
- Calcium pantothenate
Catalog No.:BCN8503
CAS No.:137-08-6
- Lidocaine
Catalog No.:BCC1084
CAS No.:137-58-6
- L-Ascorbyl 6-palmitate
Catalog No.:BCC4915
CAS No.:137-66-6
Stimulatory antibody-induced activation and selective translocation of protein kinase C isoenzymes in human platelets.[Pubmed:7487874]
Biochem J. 1995 Oct 15;311 ( Pt 2):401-6.
A novel stimulatory monoclonal antibody (Mab) termed Mab.F11 induces granular secretion and subsequent aggregation of human platelets. Mab.F11 recognizes a unique 32 and 35 kDa protein duplex on the platelet membrane surface, called the F11 receptor; binding of Mab.F11 to its receptor results in increased intracellular phosphorylation of P47, the known protein kinase C (PKC) substrate pleckstrin. In order to determine whether the mechanism of action of Mab.F11 involves direct activation of PKC, two types of functional assays for measuring PKC activity were performed. Measurement of PKC activity in digitonin-permeabilized platelets revealed that Mab.F11 produced a rapid, 2-3 fold increase in the control value in the phosphorylation of the PKC peptide substrate, PKC(19-31) Ser25. The increase in PKC activity induced by Mab.F11 was found to be associated with the platelet membrane; a 1.6-fold control value increase in membrane PKC activity occurred rapidly, within 10 s of the addition of Mab.F11. The translocation from the cytoplasm to the membrane induced by Mab.F11 in PKC isoenzymes alpha and zeta was reversible, whereas translocation of the PKC isoenzymes delta, beta, eta' and theta was irreversible, with PKC levels remaining elevated in the membrane for at least 15 min. Taken together, our results demonstrate that in the initial stages of platelet activation by this stimulatory antibody, the enhanced membrane PKC activity reflects the presence of all six isoenzymes. At later stages, PKC activity is reflective of four isoenzymes. These results demonstrate that separate groups of PKC isoenzymes must be involved in different aspects of platelet activation. The long lag period and prolonged activation time of platelets by Mab.F11 renders this agonist most suitable for identifying the isoenzymes and their specific endogenous protein substrates involved in platelet secretion and aggregation induced by platelet membrane protein antibodies.
Signal transduction mechanism in human neutrophil: comparative study between the zeta and beta-protein kinase isotypes.[Pubmed:10724343]
Mol Cell Biochem. 2000 Jan;203(1-2):143-51.
Role of protein kinase C (PKC) isotypes in the regulation of neutrophil function are not clearly known. In the present study we purified the beta-PKC and zeta-PKC isotypes from human neutrophil. Both the isotypes are immunoreactive only to their respective antibodies. Zeta-PKC was further confirmed by RT-PCR using specific primer. Co-factor requirements for both the kinases were found to be different when DG and ceramide were used as second messenger. Selective substrate specificities were determined for both beta and zeta-PKC using isotype specific pseudosubstrates viz., [Ser25]PKC[19-31] and [Ser119]PKC[113-130] respectively. Endogenous protein phosphorylation by purified beta-PKC and zeta-PKC showed their functional differences in neutrophil. Beta-PKC phosphorylated 13, 15, 19, 33, 36, 47, 80 and 92 kDa proteins and zeta-PKC phosphorylated 19, 22, 42, 47, 75 and 87 kDa proteins, only exception was the phosphorylation of 47 kDa protein which had been phosphorylated by both the kinases. Differences in phosphorylation between beta-PKC and zeta-PKC clearly indicate the selective role for these PKC isotypes in the activation sequences of neutrophil.
Protein kinase C controls the priming step of regulated exocytosis in adrenal chromaffin cells.[Pubmed:9619293]
Cell Mol Neurobiol. 1998 Aug;18(4):379-90.
1. To investigate the mechanism whereby protein kinase C enhances secretory function in adrenal chromaffin cells, we examined the effects of 12-O-tetradecanoylphorbor-13-acetate (TPA) on Ca(2+)-induced catecholamine release from digitonin-permeabilized cells, resolving the release into a MgATP-dependent priming step and a MgATP-independent Ca(2+)-triggered step. Treatment with TPA selectively potentiated the priming activity of MgATP, with little increase in the MgATP-independent release. The potentiation by TPA of the MgATP-dependent priming was blocked by [Ser25]protein kinase C(19-31), a specific substrate of protein kinase C. Go 6976, an inhibitor selective for protein kinase C alpha and beta isoforms, also blocked the potentiation by TPA. These results suggest that activation of protein kinase C, probably the alpha isoform, potentiates the MgATP-dependent priming step. 2. The antibody raised against GAP-43, a known substrate of protein kinase C, also potentiated the MgATP-dependent priming. The effect of TPA and that of the anti-GAP-43 antibody were not additive. Calmodulin, which binds to GAP-43 and inhibits its phosphorylation by protein kinase C, abolished the effect of TPA. Thus, the present results suggest that protein kinase C potentiates MgATP-dependent priming, at least in part, through phosphorylation of GAP-43.
Inhibitory effect of annexin V on protein kinase C activity in mesangial cell lysates.[Pubmed:7588728]
Eur J Biochem. 1995 Sep 15;232(3):865-72.
Annexin V belongs to a large family of calcium-binding and phospholipid-binding proteins and may act as an endogenous regulator of the protein kinase C (PKC) activity. This study examines the effect of annexin V on the in vitro PKC activity in cultured mesangial cells using histone H1, the peptide [Ser25]PKC-(19-31), or endogenous proteins as substrates. The SDS/PAGE pattern of 32P-labeled mesangial proteins showed that the calcium-independent PKC [(n+a)PKC] phosphorylated several proteins from 70 kDa to 40 kDa and 22 kDa to 15 kDa. Three additional proteins from 34 kDa to 29 kDa, including annexin I and its proteolytic forms, were detected after activation of calcium-dependent PKC (cPKC). Increasing concentrations of annexin V did not alter the phosphorylation of (n+a)PKC substrates. By contrast, specific phosphorylation of proteins and annexin I by cPKC, was reduced in a dose-dependent manner. Addition of high concentration of calcium and phosphatidylserine did not reverse the inhibitory effect of annexin V. Annexin V also inhibited the phosphorylation of histone H1 or peptide [Ser25]PKC-(19-31) by cPKC. Moreover, removal of annexin V from cytosols increased the annexin I phosphorylation by these isoforms. From these results, we propose that annexin V may regulate the signal-transduction pathway involving the activation of cPKC, as they act in vitro as an inhibitor of these kinases.
A rice membrane calcium-dependent protein kinase is induced by gibberellin.[Pubmed:7610167]
Plant Physiol. 1995 Jun;108(2):787-93.
A rice (Oryza sativa) seed plasma-membrane calcium-dependent serine/threonine protein kinase (CDPK) has been partially purified. Comparing results in seeds that were treated with and without the plant hormone gibberellin (GA) for 10 min showed that rice CDPK was highly induced by GA. After separating solubilized membrane proteins by sodium dodecyl sulfate-gel electrophoresis, followed by renaturation, a radiolabeled phosphoprotein band of approximately 58 kD was detected, and it was apparently produced by autophosphorylation. There are five aspects of the rice CDPK that show similarity to mammalian protein kinase C (PKC) and to other plant CDPKs: (a) Histone IIIS and PKC peptide-ser25 (19-31) are phosphorylated by rice CDPK. (b) The phosphorylation reaction is strictly dependent on calcium. (c) The activity of the rice CDPK is inhibited by either staurosporine or the PKC inhibitory peptide (19-36). (d) Addition of calmodulin has no effect on the activity of the enzyme; however, the CDPK is inhibited by the calmodulin antagonists trifluoperazine and W-7. (e) The rice CDPK reacts with a mammalian anti-PKC antibody in immunoblotting analysis. However, there is one major difference between the rice CDPK and other CDPKs: the rice CDPK is induced by GA, whereas no mammalian PKC or other plant CDPKs are known to be induced by any hormone.


