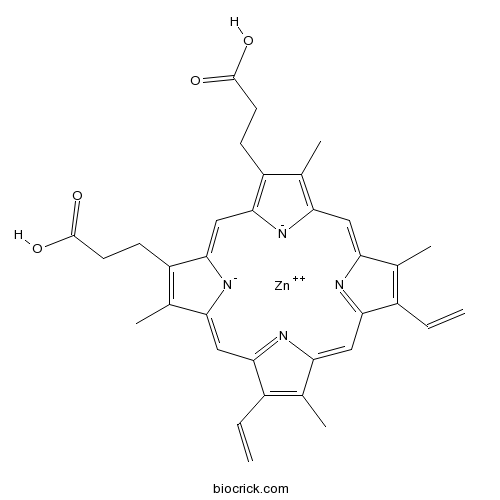
Zinc protoporphyrin IXHeme oxygenase and guanylyl cyclase inhibitor CAS# 15442-64-5 |
- Zileuton sodium
Catalog No.:BCC4216
CAS No.:118569-21-4
- Sivelestat sodium salt
Catalog No.:BCC2368
CAS No.:150374-95-1
- SC 57461A
Catalog No.:BCC2348
CAS No.:423169-68-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 15442-64-5 | SDF | Download SDF |
| PubChem ID | 455799 | Appearance | Powder |
| Formula | C34H32N4O4Zn | M.Wt | 626.03 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (79.87 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 7,12-Diethenyl-3,8,13,17-tetramethy | ||
| SMILES | [Zn++]|1|[N-]2C/3=CC4=NC(=C/c5[n-]|1c(/C=C/6N=C(/C=C2/C(=C3C)CCC(O)=O)C(=C6C)CCC(O)=O)c(C=C)c5C)C(=C4C)C=C | ||
| Standard InChIKey | FUTVBRXUIKZACV-RGUWPKJZSA-L | ||
| Standard InChI | InChI=1S/C34H34N4O4.Zn/c1-7-21-17(3)25-13-26-19(5)23(9-11-33(39)40)31(37-26)16-32-24(10-12-34(41)42)20(6)28(38-32)15-30-22(8-2)18(4)27(36-30)14-29(21)35-25;/h7-8,13-16H,1-2,9-12H2,3-6H3,(H4,35,36,37,38,39,40,41,42);/q;+2/p-2/b25-13?,26-13-,27-14?,28-15-,29-14-,30-15?,31-16-,32-16?; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of heme oxygenase, which generates the putative neurotransmitter CO. |

Zinc protoporphyrin IX Dilution Calculator

Zinc protoporphyrin IX Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5974 mL | 7.9868 mL | 15.9737 mL | 31.9474 mL | 39.9342 mL |
| 5 mM | 0.3195 mL | 1.5974 mL | 3.1947 mL | 6.3895 mL | 7.9868 mL |
| 10 mM | 0.1597 mL | 0.7987 mL | 1.5974 mL | 3.1947 mL | 3.9934 mL |
| 50 mM | 0.0319 mL | 0.1597 mL | 0.3195 mL | 0.6389 mL | 0.7987 mL |
| 100 mM | 0.016 mL | 0.0799 mL | 0.1597 mL | 0.3195 mL | 0.3993 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Abiraterone Acotate
Catalog No.:BCN2184
CAS No.:154229-18-2
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- LY 303511
Catalog No.:BCC1715
CAS No.:154447-38-8
- Methyl 5-O-feruloylquinate
Catalog No.:BCN3402
CAS No.:154461-64-0
- Sinapaldehyde glucoside
Catalog No.:BCN1689
CAS No.:154461-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- SB 204990
Catalog No.:BCC6342
CAS No.:154566-12-8
- Stauprimide
Catalog No.:BCC7768
CAS No.:154589-96-5
- Efavirenz
Catalog No.:BCC4135
CAS No.:154598-52-4
Hierarchical coassembly of DNA-triptycene hybrid molecular building blocks and zinc protoporphyrin IX.[Pubmed:27335759]
Beilstein J Nanotechnol. 2016 May 12;7:697-707.
Herein, we describe the successful construction of composite DNA nanostructures by the self-assembly of complementary symmetrical 2,6,14-triptycenetripropiolic acid (TPA)-DNA building blocks and Zinc protoporphyrin IX (Zn PpIX). DNA-organic molecule scaffolds for the composite DNA nanostructure were constructed through covalent conjugation of TPA with 5'-C12-amine-terminated modified single strand DNA (ssDNA) and its complementary strand. The repeated covalent conjugation of TPA with DNA was confirmed by using denaturing polyacrylamide gel electrophoresis (PAGE), reverse-phase high-performance liquid chromatography (RP-HPLC) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF). The biologically relevant photosensitizer Zn PpIX was used to direct the hybridization-mediated self-assembly of DNA-TPA molecular building blocks as well as a model guest molecule within the DNA-TPA supramolecular self-assembly. The formation of fiber-like composite DNA nanostructures was observed. Native PAGE, circular dichroism (CD) and atomic force microscopy (AFM) have been utilized for analyzing the formation of DNA nanofibers after the coassembly. Computational methods were applied to discern the theoretical dimension of the DNA-TPA molecular building block of the nanofibers. A notable change in photocatalytic efficiency of Zn PpIX was observed when it was inside the TPA-DNA scaffold. The significant increase in ROS generation by Zn PpIX when trapped in this biocompatible DNA-TPA hybrid nanofiber may be an effective tool to explore photodynamic therapy (PDT) applications as well as photocatalytic reactions.
Mechanisms of Vesicular Stomatitis Virus Inactivation by Protoporphyrin IX, Zinc-Protoporphyrin IX, and Mesoporphyrin IX.[Pubmed:28348154]
Antimicrob Agents Chemother. 2017 May 24;61(6). pii: AAC.00053-17.
Virus resistance to antiviral therapies is an increasing concern that makes the development of broad-spectrum antiviral drugs urgent. Targeting of the viral envelope, a component shared by a large number of viruses, emerges as a promising strategy to overcome this problem. Natural and synthetic porphyrins are good candidates for antiviral development due to their relative hydrophobicity and pro-oxidant character. In the present work, we characterized the antiviral activities of protoprophyrin IX (PPIX), Zn-protoporphyrin IX (ZnPPIX), and mesoporphyrin IX (MPIX) against vesicular stomatitis virus (VSV) and evaluated the mechanisms involved in this activity. Treatment of VSV with PPIX, ZnPPIX, and MPIX promoted dose-dependent virus inactivation, which was potentiated by porphyrin photoactivation. All three porphyrins inserted into lipid vesicles and disturbed the viral membrane organization. In addition, the porphyrins also affected viral proteins, inducing VSV glycoprotein cross-linking, which was enhanced by porphyrin photoactivation. Virus incubation with sodium azide and alpha-tocopherol partially protected VSV from inactivation by porphyrins, suggesting that singlet oxygen ((1)O2) was the main reactive oxygen species produced by photoactivation of these molecules. Furthermore, (1)O2 was detected by 9,10-dimethylanthracene oxidation in photoactivated porphyrin samples, reinforcing this hypothesis. These results reveal the potential therapeutic application of PPIX, ZnPPIX, and MPIX as good models for broad antiviral drug design.
Probing the Highly Efficient Electron Transfer Dynamics between Zinc Protoporphyrin IX and Sodium Titanate Nanosheets.[Pubmed:27537491]
J Phys Chem A. 2016 Sep 15;120(36):7121-9.
Sodium titanate nanosheets (NaTiO2 NS) have been prepared by a new method and completely characterized by TEM, SEM, XRD, EDX, and XPS techniques. The sensitization of nanosheets is carried out with Zn protoporphyrin IX (ZnPPIX). The emission intensity of ZnPPIX is quenched by NaTiO2 NS, and the dominant process for this quenching has been attributed to the process of photoinduced electron injection from excited ZnPPIX to the nanosheets. Time resolved fluorescence measurement was used to elucidate the process of electron injection from the singlet state of ZnPPIX to the conduction band of NaTiO2 NS. Electron injection from the dye to the semiconductor is very fast (ket approximately 10(11) s(-1)), much faster than previously reported rates. The large two-dimensional surface offered by the NaTiO2 NS for interaction with the dye and the favorable driving force for electron injection from ZnPPIX to NaTiO2 NS (DeltaGinj = -0.66 V) are the two important factors responsible for such efficient electron injection. Thus, NaTiO2 NS can serve as an effective alternative to the use of TiO2 nanoparticles in dye sensitized solar cells (DSSCs).
A comparative study of zinc protoporphyrin IX-forming properties of animal by-products as sources for improving the color of meat products.[Pubmed:25441257]
Anim Sci J. 2015 May;86(5):547-52.
The objective of this study was to obtain fundamental data for improving the color of meat products by using animal by-products. We investigated Zinc protoporphyrin IX (ZnPP)-forming properties of various internal organs from pigs and chickens. ZnPP was formed in the liver, heart and kidney, whereas the porcine spleen and bile, which are involved in the metabolism of heme, did not have ZnPP-forming properties. The optimum pH values were different among the internal organs and the ZnPP-forming properties of porcine organs were better than those of chicken organs. The porcine liver showed the greatest ZnPP-forming properties among all of the internal organs investigated in this study. The optimum pH value for ZnPP formation in the liver was lower than that of skeletal muscle. Oxygen did not inhibit the formation of ZnPP in the liver, unlike in skeletal muscle. Animal by-products such as the liver have good ability for the formation of ZnPP and might be useful for improving the color of meat products.
Pitfalls using metalloporphyrins in carbon monoxide research.[Pubmed:9226997]
Trends Pharmacol Sci. 1997 Jun;18(6):193-5.
The proposal that endogenously produced carbon monoxide (CO) may act as a biological messenger has remained controversial. Carbon monoxide is generated by haem oxygenase isoenzymes in the degradation of haem-containing molecules. Certain metalloporphyrins, which are inhibitors of haem oxygenase, have been widely used as pharmacological tools in order to establish a messenger role for CO in the brain and periphery. However, increasing evidence shows that many metalloporphyrins are also associated with a large range of undesired effects, which make the interpretation of results using such compounds very uncertain. In this article, Lars Grundemar and Lars Ny evaluate the properties and describe the nonselective effect profile of such metalloporphyrins.
Metalloporphyrins inhibit nitric oxide-dependent cGMP formation in vivo.[Pubmed:7522180]
Eur J Pharmacol. 1994 May 17;267(3):263-7.
Sodium nitroprusside produced a dose-dependent increase in extracellular levels of cGMP in the cerebellar cortex in vivo. This was independent of nitric oxide synthase activity. The metalloporphyrins zinc-protoporphyrin-IX, tin-protoporphyrin-IX and zinc-deuteroporphyrin-IX,2,4-bis glycol prevented the increase in cGMP in the cerebellar cortex produced by sodium nitroprusside. At high doses, tin-protoporphyrin-IX also decreased the basal extracellular levels of cGMP. These drugs had no effect on nitric oxide synthase activity. We conclude that the neuropharmacological effects of metalloporphyrins may result from their direct inhibition of soluble guanylyl cyclase, rather than from an effect on carbon monoxide synthesis.
Carbon monoxide: a putative neural messenger.[Pubmed:7678352]
Science. 1993 Jan 15;259(5093):381-4.
Carbon monoxide, an activator of guanylyl cyclase, is formed by the action of the enzyme heme oxygenase. By in situ hybridization in brain slices, discrete neuronal localization of messenger RNA for the constitutive form of heme oxygenase throughout the brain has been demonstrated. This localization is essentially the same as that for soluble guanylyl cyclase messenger RNA. In primary cultures of olfactory neurons, zinc protoporphyrin-9, a potent selective inhibitor of heme oxygenase, depletes endogenous guanosine 3',5'-monophosphate (cGMP). Thus, carbon monoxide, like nitric oxide, may be a physiologic regulator of cGMP. These findings, together with the neuronal localizations of heme oxygenase, suggest that carbon monoxide may function as a neurotransmitter.
Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase.[Pubmed:2890093]
Mol Pharmacol. 1987 Oct;32(4):497-504.
Carbon monoxide (CO) inhibits human platelet aggregation triggered with threshold levels of agonists like arachidonate, ADP, collagen, thrombin, or the prostaglandin endoperoxide analogue U46619. This inhibition is counteracted by illumination with light above 400 nm indicating the involvement of a ferrous hemoprotein. An earlier suggestion that the mechanism of CO inhibition involves the cytochrome P450 protein thromboxane A2 synthase was ruled out as well as the involvement of the iron containing enzymes like cyclooxygenase or 12-lipoxygenase. In the presence of CO, no arachidonate was released from phospholipids, no increase of intracellular calcium levels was observed, and phospholipase C was not activated suggesting that the transducing mechanisms from the receptors to phospholipase C was effected in the presence of CO. cAMP levels were also unchanged but cGMP levels showed an increase of about 30%. By comparison with the guanylate cyclase stimulator nitroprusside, it was shown that such levels could block aggregation. In a 10,000 X g supernatant, CO enhanced guanylate cyclase activity 4-fold, supporting the view that CO acts by increasing platelet cGMP levels. With respect to the mechanism of guanylate cyclase action, the binding of CO to the regulatory subunit of guanylate cyclase must be responsible for the observed activation. It is concluded that cGMP is an important feedback regulator of the Pl response and that already a 25% increase in its steady state levels can cause inhibition of platelet aggregation.


