Abiraterone AcotateCytochrome p450 17a1 inhibitor CAS# 154229-18-2 |
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- VT-464
Catalog No.:BCC5398
CAS No.:1610537-15-9
- TOK-001
Catalog No.:BCC3910
CAS No.:851983-85-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 154229-18-2 | SDF | Download SDF |
| PubChem ID | 132970 | Appearance | Powder |
| Formula | C26H33NO2 | M.Wt | 391.55 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble to 28 mg/mL (71.51 mM) in Ethanol | ||
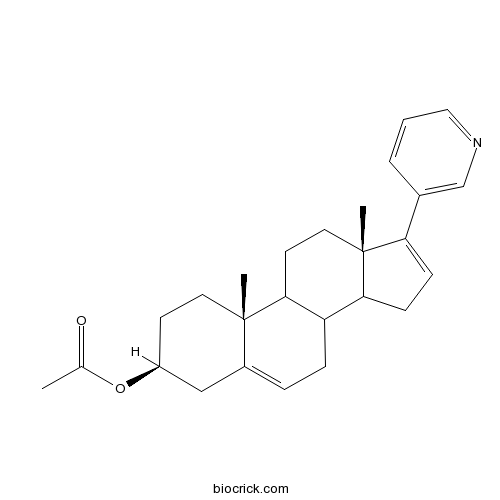
| Chemical Name | [(3S,10R,13S)-10,13-dimethyl-17-pyridin-3-yl-2,3,4,7,8,9,11,12,14,15-decahydro-1H-cyclopenta[a]phenanthren-3-yl] acetate | ||
| SMILES | CC(=O)OC1CCC2(C3CCC4(C(C3CC=C2C1)CC=C4C5=CN=CC=C5)C)C | ||
| Standard InChIKey | UVIQSJCZCSLXRZ-HMMZIKKISA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Abiraterone acetate shows antitumour activity against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100), it can significantly prolong overall survival in patients with metastatic castration-resistant prostate cancer who have progressed after docetaxel treatment. 2. Abiraterone acetate is a potent, selective, and orally available inhibitor of CYP17, the key enzyme in androgen and estrogen biosynthesis. 3. Abiraterone acetate achieves sustained suppression of testosterone in both blood and bone marrow aspirate to less than picograms-per-milliliter levels. |
| Targets | P450 (e.g. CYP17) |

Abiraterone Acotate Dilution Calculator

Abiraterone Acotate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.554 mL | 12.7698 mL | 25.5395 mL | 51.079 mL | 63.8488 mL |
| 5 mM | 0.5108 mL | 2.554 mL | 5.1079 mL | 10.2158 mL | 12.7698 mL |
| 10 mM | 0.2554 mL | 1.277 mL | 2.554 mL | 5.1079 mL | 6.3849 mL |
| 50 mM | 0.0511 mL | 0.2554 mL | 0.5108 mL | 1.0216 mL | 1.277 mL |
| 100 mM | 0.0255 mL | 0.1277 mL | 0.2554 mL | 0.5108 mL | 0.6385 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Abiraterone acetate is the 3β-acetate form of its active component abiraterone, a potent inhibitor of androgen biosynthesis. Abiraterone, a pregnenolone-derived 3-pyridyl steroidal compound, potently and irreversibly inhibits cytochrome P450 17 alpha-hydroxylase (CYP17), an important enzyme involved in the synthesis of androgen and cortisol, through a covalent binding mechanism with a value of 50% inhibition concentration IC50 of 72 nM, which is 10 to 30 times greater in potency, due to the 3-pyridyl substitution in its chemical structure, than the inhibition by ketoconazole. In order to improve the low solubility of abirateone, abiraterone acetate has been developed and used for the treatment of castration-resistant prostate cancer (CRPC).
Reference
Charles J Ryan and Michael L Cheng. Abiraterone acetate for the treatment of prostate cancer. Expert Opin. Pharmacother. (2013) 14 (1): 91-96
Tadas S. Vasaitis, Robert D. Bruno and Vincent C.O. Njar. CYP17 inhibitors for prostate cancer therapy. Journal of Steroid Biochemistry & Molecular Biology 125 (2011) 23-31
Guru Sonpavde, Gerhardt Attard, Joaquim Bellmunt, Malcolm D. Mason, Bernard Malavaud, Bertrand Tombal and Cora N. Sternberg. The role of abiraterone acetate in the management of prostate cancer: a critical analysis of the literature. EUROPEAN UROLOGY 60 (2011) 270-278
- Clasto-Lactacystin β-lactone
Catalog No.:BCC1224
CAS No.:154226-60-5
- N-[2-Isopropylthiazol-4-ylmethyl(methyl)carbamoyl]-L-valine
Catalog No.:BCC9067
CAS No.:154212-61-0
- YM 90K hydrochloride
Catalog No.:BCC7455
CAS No.:154164-30-4
- DMAB-anabaseine dihydrochloride
Catalog No.:BCC7301
CAS No.:154149-38-9
- PD 144418 oxalate
Catalog No.:BCC7429
CAS No.:154130-99-1
- 4-Hydroxy-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8707
CAS No.:154127-42-1
- 2-(3-Methoxypropyl)-4-oxo-3,4-dihydro-2H-thieno[3,2-e][1,2]thiazine-6-sulfonamide 1,1-dioxide
Catalog No.:BCC8480
CAS No.:154127-41-0
- ((2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)methyl)triphenylphosphonium bromide
Catalog No.:BCC8373
CAS No.:154057-58-6
- 3-(Bromomethyl)-2-cyclopropyl-4-(4'-fluorophenyl)quinoline
Catalog No.:BCC8591
CAS No.:154057-56-4
- Isonormangostin
Catalog No.:BCN1687
CAS No.:15404-80-5
- Fuscaxanthone C
Catalog No.:BCN3885
CAS No.:15404-76-9
- Marimastat
Catalog No.:BCC2118
CAS No.:154039-60-8
- Abiraterone
Catalog No.:BCC2259
CAS No.:154229-19-3
- Ampalex
Catalog No.:BCC1359
CAS No.:154235-83-3
- CB-5083
Catalog No.:BCC6528
CAS No.:1542705-92-9
- Echistatin, α1 isoform
Catalog No.:BCC5988
CAS No.:154303-05-6
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Methyl 3-O-feruloylquinate
Catalog No.:BCN3403
CAS No.:154418-15-2
- 5,5'-Dimethoxylariciresinol 4-O-glucoside
Catalog No.:BCN1556
CAS No.:154418-16-3
- Zinc protoporphyrin IX
Catalog No.:BCC6775
CAS No.:15442-64-5
- Lysicamine
Catalog No.:BCN6523
CAS No.:15444-20-9
- Pramanicin
Catalog No.:BCN1853
CAS No.:154445-05-3
- Fmoc-Arg(Pbf)-OH
Catalog No.:BCC3040
CAS No.:154445-77-9
- NU 7026
Catalog No.:BCC3933
CAS No.:154447-35-5
Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone.[Pubmed:22184395]
J Clin Oncol. 2012 Feb 20;30(6):637-43.
PURPOSE: Persistent androgen signaling is implicated in castrate-resistant prostate cancer (CRPC) progression. This study aimed to evaluate androgen signaling in bone marrow-infiltrating cancer and testosterone in blood and bone marrow and to correlate with clinical observations. PATIENTS AND METHODS: This was an open-label, observational study of 57 patients with bone-metastatic CRPC who underwent transiliac bone marrow biopsy between October 2007 and March 2010. Patients received oral abiraterone acetate (1 g) once daily and prednisone (5 mg) twice daily. Androgen receptor (AR) and CYP17 expression were assessed by immunohistochemistry, testosterone concentration by mass spectrometry, AR copy number by polymerase chain reaction, and TMPRSS2-ERG status by fluorescent in situ hybridization in available tissues. RESULTS: Median overall survival was 555 days (95% CI, 440 to 965+ days). Maximal prostate-specific antigen decline >/= 50% occurred in 28 (50%) of 56 patients. Homogeneous, intense nuclear expression of AR, combined with >/= 10% CYP17 tumor expression, was correlated with longer time to treatment discontinuation (> 4 months) in 25 patients with tumor-infiltrated bone marrow samples. Pretreatment CYP17 tumor expression >/= 10% was correlated with increased bone marrow aspirate testosterone. Blood and bone marrow aspirate testosterone concentrations declined to less than picograms-per-milliliter levels and remained suppressed at progression. CONCLUSION: The observed pretreatment androgen-signaling signature is consistent with persistent androgen signaling in CRPC bone metastases. This is the first evidence that abiraterone acetate achieves sustained suppression of testosterone in both blood and bone marrow aspirate to less than picograms-per-milliliter levels. Potential admixture of blood with bone marrow aspirate limits our ability to determine the origin of measured testosterone.
Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer.[Pubmed:19470933]
J Clin Oncol. 2009 Aug 10;27(23):3742-8.
PURPOSE: It has been postulated that castration-resistant prostate cancer (CRPC) commonly remains hormone dependent. Abiraterone acetate is a potent, selective, and orally available inhibitor of CYP17, the key enzyme in androgen and estrogen biosynthesis. PATIENTS AND METHODS: This was a phase I/II study of abiraterone acetate in castrate, chemotherapy-naive CRPC patients (n = 54) with phase II expansion at 1,000 mg (n = 42) using a two-stage design to reject the null hypothesis if more than seven patients had a prostate-specific antigen (PSA) decline of > or = 50% (null hypothesis = 0.1; alternative hypothesis = 0.3; alpha = .05; beta = .14). Computed tomography scans every 12 weeks and circulating tumor cell (CTC) enumeration were performed. Prospective reversal of resistance at progression by adding dexamethasone 0.5 mg/d to suppress adrenocorticotropic hormone and upstream steroids was pursued. RESULTS: A decline in PSA of > or = 50% was observed in 28 (67%) of 42 phase II patients, and declines of > or = 90% were observed in eight (19%) of 42 patients. Independent radiologic evaluation reported partial responses (Response Evaluation Criteria in Solid Tumors) in nine (37.5%) of 24 phase II patients with measurable disease. Decreases in CTC counts were also documented. The median time to PSA progression (TTPP) on abiraterone acetate alone for all phase II patients was 225 days (95% CI, 162 to 287 days). Exploratory analyses were performed on all 54 phase I/II patients; the addition of dexamethasone at disease progression reversed resistance in 33% of patients regardless of prior treatment with dexamethasone, and pretreatment serum androgen and estradiol levels were associated with a probability of > or = 50% PSA decline and TTPP on abiraterone acetate and dexamethasone. CONCLUSION: CYP17 blockade by abiraterone acetate results in declines in PSA and CTC counts and radiologic responses, confirming that CRPC commonly remains hormone driven.
Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study.[Pubmed:22995653]
Lancet Oncol. 2012 Oct;13(10):983-92.
BACKGROUND: Abiraterone acetate improved overall survival in metastatic castration-resistant prostate cancer at a preplanned interim analysis of the COU-AA-301 double-blind, placebo-controlled phase 3 study. Here, we present the final analysis of the study before crossover from placebo to abiraterone acetate (after 775 of the prespecified 797 death events). METHODS: Between May 8, 2008, and July 28, 2009, this study enrolled 1195 patients at 147 sites in 13 countries. Patients were eligible if they had metastatic castration-resistant prostate cancer progressing after docetaxel. Patients were stratified according to baseline Eastern Cooperative Oncology Group (ECOG) performance status, worst pain over the past 24 h on the Brief Pain Inventory-Short Form, number of previous chemotherapy regimens, and type of progression. Patients were randomly assigned (ratio 2:1) to receive either abiraterone acetate (1000 mg, once daily and orally) plus prednisone (5 mg, orally twice daily) or placebo plus prednisone with a permuted block method via an interactive web response system. The primary endpoint was overall survival, analysed in the intention-to-treat population. This study is registered with ClinicalTrials.gov, number NCT00091442. FINDINGS: Of the 1195 eligible patients, 797 were randomly assigned to receive abiraterone acetate plus prednisone (abiraterone group) and 398 to receive placebo plus prednisone (placebo group). At median follow-up of 20.2 months (IQR 18.4-22.1), median overall survival for the abiraterone group was longer than in the placebo group (15.8 months [95% CI 14.8-17.0] vs 11.2 months [10.4-13.1]; hazard ratio [HR] 0.74, 95% CI 0.64-0.86; p<0.0001). Median time to PSA progression (8.5 months, 95% CI 8.3-11.1, in the abiraterone group vs 6.6 months, 5.6-8.3, in the placebo group; HR 0.63, 0.52-0.78; p<0.0001), median radiologic progression-free survival (5.6 months, 5.6-6.5, vs 3.6 months, 2.9-5.5; HR 0.66, 0.58-0.76; p<0.0001), and proportion of patients who had a PSA response (235 [29.5%] of 797 patients vs 22 [5.5%] of 398; p<0.0001) were all improved in the abiraterone group compared with the placebo group. The most common grade 3-4 adverse events were fatigue (72 [9%] of 791 patients in the abiraterone group vs 41 [10%] of 394 in the placebo group), anaemia (62 [8%] vs 32 [8%]), back pain (56 [7%] vs 40 [10%]), and bone pain (51 [6%] vs 31 [8%]). INTERPRETATION: This final analysis confirms that abiraterone acetate significantly prolongs overall survival in patients with metastatic castration-resistant prostate cancer who have progressed after docetaxel treatment. No new safety signals were identified with increased follow-up.
Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100).[Pubmed:23576708]
Ann Oncol. 2013 Jul;24(7):1807-12.
BACKGROUND: Androgen receptor (AR) signalling remains critically important in metastatic castration-resistant prostate cancer (mCRPC) as confirmed by recent phase III trials, showing a survival advantage for abiraterone acetate and enzalutamide (MDV3100). The antitumour activity of abiraterone and prednisolone in patients pre-treated with enzalutamide is as yet unknown. PATIENTS AND METHODS: We investigated the antitumour activity of abiraterone and prednisolone in patients with mCRPC who had progressed following treatment with docetaxel (Taxotere) and enzalutamide. Clinical data were retrospectively analysed for prostate-specific antigen (PSA) and RECIST responses, clinical benefit and survival. RESULTS: Thirty-eight patients were included in the analysis. The median age was 71 years (range 52-84); metastatic sites included bone disease in 37 patients (97%), lymph nodes in 15 patients (39%) and visceral disease in 10 patients (26%). Abiraterone was well tolerated. Three patients (8%) attained a PSA response, defined as >/=50% decline in PSA confirmed after >/=4 weeks, while seven patients (18%) had a >/=30% PSA decline. The median progression-free survival (PFS) was 2.7 months (95% CI 2.3-4.1). Of the 12 patients assessable radiologically, only 1 (8%) attained a confirmed partial response. CONCLUSION: Abiraterone and prednisolone have modest antitumour activities in patients with mCRPC pretreated with docetaxel and enzalutamide.


